CBSE Class 11 Chemistry Notes Chapter 10: CBSE Class 11 Chemistry Notes Chapter 10 talks about the s-Block elements. These elements are important because they help us understand the properties of alkali metals and alkaline earth metals.
The CBSE Class 11 Chemistry Notes Chapter 10 covers things like their electronic setup, where they're found, and what they're like physically and chemically. We also learn about how they react with other elements and how their properties change in a group. These elements have many practical uses, so it is important to understand them well.CBSE Class 11 Chemistry Notes Chapter 10 Overview
The Group 1 and Group 2 elements of the periodic table, sometimes known as Alkali Metals and Alkali Earth Metals, respectively, are the elements that make up the s-Block. For alkali metals, these are represented by one s-electron, and for alkali earth metals, by two s-electron. Our known for offering the greatest course materials to students in all grades. Students can better prepare for their exams by using the thorough notes on each element's properties and reactions found in our Revision Notes for Class 11 Chemistry Chapter 10.CBSE Class 11 Chemistry Notes Chapter 10 The s-Block Elements PDF
You can access the CBSE Class 11 Chemistry Notes for Chapter 10 on the s-Block Elements through the provided PDF link. These notes are a valuable resource for students studying this chapter as they cover important concepts, properties, and reactions of alkali metals and alkaline earth metals. By referring to these CBSE Class 11 Chemistry Notes Chapter 10, students can enhance their understanding of the s-Block elements and prepare effectively for their examinations.CBSE Class 11 Chemistry Notes Chapter 10 The s-Block Elements PDF
CBSE Class 11 Chemistry Notes Chapter 10 The s-Block Elements
Alkali Metals (Group 1)
Alkali metals belong to Group 1 of the periodic table, which includes elements like lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These metals are highly reactive and are typically soft, silvery-white metals. They have low melting and boiling points compared to other metals and are excellent conductors of heat and electricity. Alkali metals readily lose their outermost electron to form ions with a +1 oxidation state, making them highly reactive with water and oxygen. Their reactivity increases down the group, with francium being the most reactive alkali metal. Alkali metals are widely used in various applications, including the production of alkali metal compounds, batteries, and industrial processes.Electrode Potential
The electrode potential of a metal in water gauges its inclination to donate electrons. Standard electrode potential is determined when the concentration of metal ions equals one. Lithium, despite having the highest ionization potential, also exhibits the highest electrode potential due to its significant hydration energy.Hydration of Ions
Ions possess varying degrees of hydration depending on their size. Consequently, as one moves down the group from Li+ to Cs+, the degree of hydration decreases, leading to a decline in electrical conductivity as hydration increases.Lattice Energy
Ionic solids constitute alkali metal salts, and the lattice energy of these salts with a common anion diminishes as the group descends.Solubility in Liquid Ammonia
In dilute alkali metal solutions in liquid ammonia, the predominant species are solvated metal ions and solvated electrons. The presence of solvated electrons allows these solutions to conduct electricity, and their paramagnetic nature arises from the inclusion of free electrons. The blue coloration of the solution fades over time due to the formation of metal amide.Basic Nature, Ionic Nature of the Oxides
Basic Nature:
The oxides of alkali metals are predominantly basic in nature. They readily react with water to form hydroxides, leading to an increase in the concentration of hydroxide ions in the solution. This property indicates their ability to accept protons, thus exhibiting basic behavior.Ionic Nature:
Alkali metal oxides are primarily ionic compounds composed of metal cations and oxide anions. Due to the large difference in electronegativity between the metal and oxygen, electrons are transferred from the metal atoms to the oxygen atoms, resulting in the formation of ions. This ionic character contributes to their high melting and boiling points, as well as their tendency to form stable crystal lattices.Down’s Process
These days, Down's method is used to create the metal. It involves electrolyzing fused sodium chloride including calcium chloride and potassium fluoride at about 600 degrees Celsius using iron as a cathode and graphite as an anode. The cell consists of a steel tank lined with heat-resistant bricks. A cylindrical iron cathode surrounds a circular graphite anode that is positioned in the centre of the cell. The anode and cathode are separated by a steel gauze cylinder, which permits fused charge to flow through. The anode is shielded and an exit for chlorine gas is provided by a steel canopy in the form of a dome. The molten metal rises and flows into the kerosene receiver after being released at the cathode.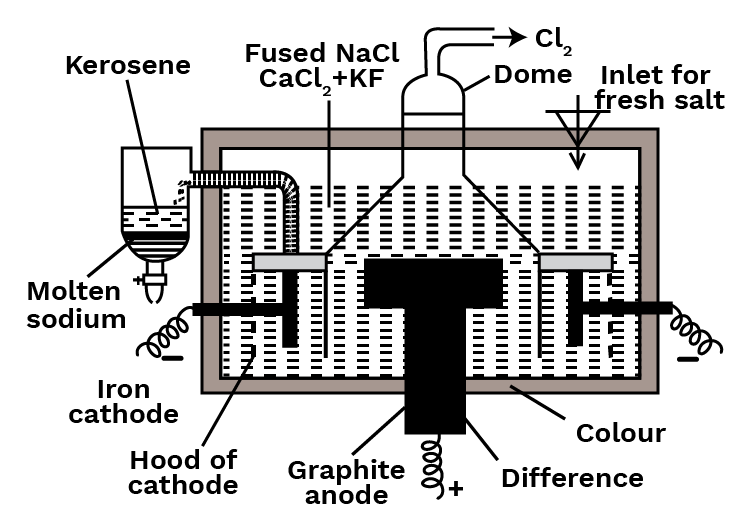
Compounds of Alkali Metal
Hydroxides: Alkali metals readily react with water to form hydroxides, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH). These hydroxides are strong bases and are commonly used in various industrial processes and chemical reactions.
Halides: Alkali metals form halides, including fluorides, chlorides, bromides, and iodides. These compounds are highly ionic and soluble in water. For example, sodium chloride (NaCl) and potassium iodide (KI) are common halides of alkali metals.
Carbonates and Bicarbonates: Alkali metals react with carbon dioxide to form carbonates (e.g., sodium carbonate, Na2CO3) and bicarbonates (e.g., sodium bicarbonate, NaHCO3). These compounds are important in various applications, such as in baking and as antacids.
Nitrates: Alkali metals form nitrates, such as sodium nitrate (NaNO3) and potassium nitrate (KNO3). These compounds are commonly used in fertilizers and as oxidizing agents in various chemical processes.
Sulfates: Alkali metals react with sulfuric acid to form sulfates, such as sodium sulfate (Na2SO4) and potassium sulfate (K2SO4). These compounds have various industrial applications, including in the manufacture of detergents and as electrolytes in batteries.
Oxides: Alkali metals react with oxygen to form oxides, such as sodium oxide (Na2O) and potassium oxide (K2O). These compounds are basic in nature and react with water to form hydroxides.
CBSE Class 11 Chemistry Notes Chapter 10 FAQs
Are these notes suitable for CBSE board exams?
Can these notes be used for quick revision before exams?
Can these notes be used by students of other education boards besides CBSE?