
CBSE Class 11 Chemistry Notes Chapter 5: As you are all aware, matter can exist in three various states: solid, liquid, or gaseous. Standard 11 expands on this concept by discussing the concept of States of Matter. Therefore, to get an advantage over your peers, you will need to work hard and use resources found online, such as our notes for Class 11 Chemistry Chapter 5. Access the States of Matter Class 11 notes on our official website, completely free of charge.
Put it anywhere convenient for you, whether it's on your tablet, laptop, or phone. This is a useful tool that will familiarise you with the fundamental ideas required for exam preparation for Class 11 and beyond.CBSE Class 11 Chemistry Notes Chapter 5 PDF
The three categories of matter—solids, liquids, and gases—are covered in Chemistry Chapter 5 States of Matter. All of the Class 11 Chemistry Chapter 5 Notes are available to students. Subject matter specialists have carefully crafted these notes so that any student can use them to study and fully understand the concepts.CBSE Class 11 Chemistry Notes Chapter 5 PDF
CBSE Class 11 Chemistry Notes Chapter 5
Matter is the substance that has mass and takes up space. Matter is made up of molecules and atoms. Its chemical and physical characteristics are diverse. There are three categories for matter: solid, liquid, and gas. Their physical qualities arise from variations in the force of interaction that exists within them. Solids interact with the world with the most force, while gases interact with it the least.Intermolecular Forces
Intermolecular forces are the forces of attraction that exist between molecules, whether they be solid, liquid, or gas. Dipole-dipole, dipole-induced dipole, and dispersion forces add together to form van der Waal forces. Van der Waal forces do not include ion-dipole and ion-induced dipole. The strongest attraction is that of hydrogen bonds.The Different Types of Intermolecular Forces are
Dipole-Dipole Interaction
Dipole-dipole interactions are seen in polar compounds. Their dipole moments are constant. The molecule attracts both the positive and negative poles. Take HCl, for instance. Cl is more electronegative than hydrogen in the HCl molecule. As a result, the hydrogen atom gains a positive charge whereas the chlorine atom gains a negative charge. Dipole-dipole contact therefore occurs between them.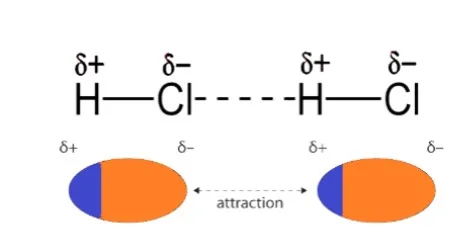
Ion-Dipole Interaction
The attraction between the cation, anion, and a polar molecule is known as ion-dipole interaction. For example, NaCl .
The polar water molecules are attracted towards N a + and C l − on dissolving NaCl in water.
Ion-induced Dipole Interaction
Dipole Induced Dipole Interaction
London forces or Dispersion forces
State of a Gas and State Variable
The physical condition of the system is the state of a gas. The variables which are used to denote the physical condition of a gas are known as state variables. They are pressure, volume and temperature ( P , V and T ) .Pressure
Pressure is the force applied to an item per unit area. Always apply force in a direction perpendicular to the object. The pascal is the unit of pressure. Numerous instruments are available for measuring pressure. Pressurisation can be measured with a manometer or barometer.Volume
For rigid containers, the volume of the gas is equal to the volume of the container. In non-rigid containers, the number of moles and additional state functions are used to calculate the gas volume.Temperature
Temperature is a physical term that describes how much heat a gas contains. When the temperature of the gas is the same as the surrounding air, no heat is transferred into or out of the gas. The gas's temperature is determined using a thermometer. Fahrenheit, Kelvin, and Celsius are the temperature units.Avogadro’s Law
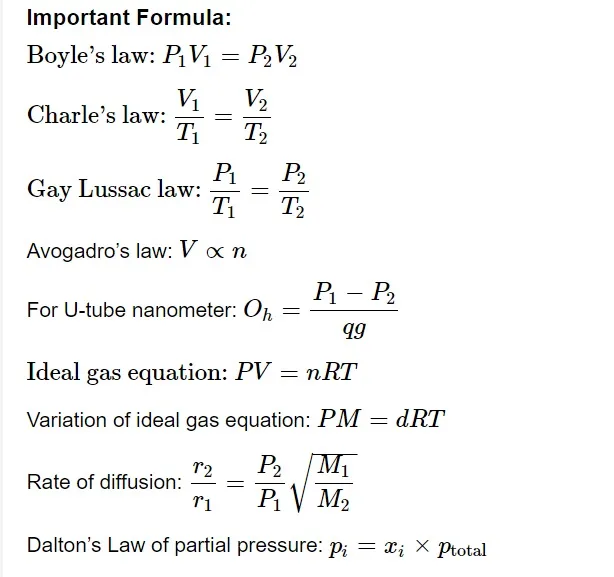
It provides the relationship between gas volume and quantity. It asserts that there are the same number of molecules in all gases with similar volumes under the same pressure and temperature conditions.
Graham’s Law of Diffusion
Diffusion of gases refers to the intermixing of gases. Even in the absence of pressure differences, gases can combine. If there is a greater pressure differential between the gases, the diffusion process proceeds more quickly. Small apertures allow gas to pass through because of pressure differences. Effusion is the gas flow. Graham's law of diffusion states that there are two variables that affect the rate of diffusion. Gas's molecular weight and partial pressure. The partial pressure and the square root of the gas's molar mass are inversely related to the rate of diffusion, which is directly proportional to both.Real Gases
When considering real gases, the assumption made for ideal gases is no longer applicable. (i) We believed that there are no molecular interactions in an ideal gas. (ii) The volume of a gas's molecules is insignificant in relation to the volume of gases as a whole. In actual gases, the molecule interaction cannot be disregarded. They are as follows: There are long-range attractive forces and short-range repulsive forces. When two real gases are far apart, the interaction forces between them become insignificant. However, as the molecules get closer together, attractive forces begin to form. The molecules begin to reject one another as they get closer.Measurement of the Pressure of the Gas
A "barometer" is a common device used to measure a gas's pressure. In a mercury barometer, the atmospheric pressure is measured by the height of a mercury column supported in a sealed glass tube. There are several ways to measure a gas's pressure. Pressure is most commonly measured in terms of height. Assume a liquid of density d 𝑑 is filled in a tube with a cross-sectional area A 𝐴 of height h ℎ . A vacuum has been placed over it. The liquid exerts pressure on the bottom of a container due to gravity.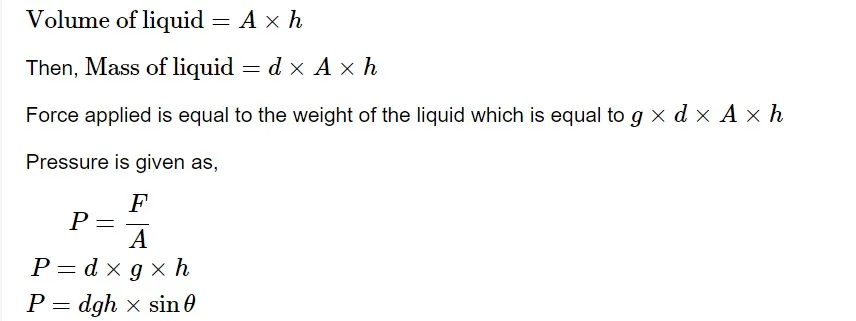
Van Der Waals Equation
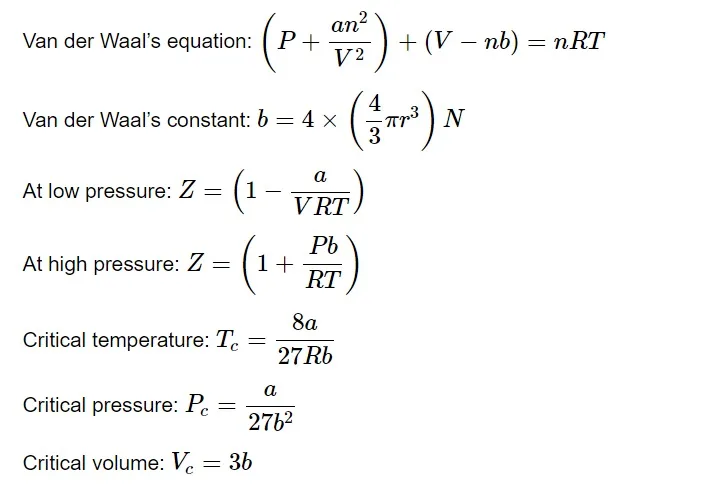
After correction of pressure and volume terms, the ideal gas equation can be formulated as,
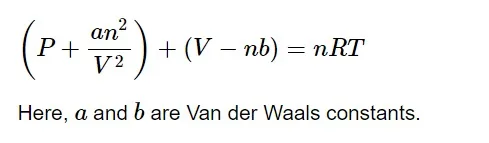
a 𝑎 is the attractive force between the gases. As the attractive forces between the molecules increases, the value of a 𝑎 increases. Whereas, b 𝑏 is the volume occupied by the molecule.
The Van der Waal constant a 𝑎 is always greater than b 𝑏 for a given gas.
The higher the value of a 𝑎 , the more easily liquefaction can occur.
Benefits of CBSE Class 11 Chemistry Notes Chapter 5
CBSE Class 11 Chemistry Notes Chapter 5 FAQs
What is the chapter 5 of class 11 chemistry?
What happens if you fail in chemistry in class 11 CBSE?
Which is the hardest chapter in chemistry class 11?










