
CBSE Class 11 Chemistry Notes Chapter 4: Most students believe that Chemistry in Class 11 is difficult and that they cannot get good grades in this subject, yet this doesn't seem to be the case. Good grades in Chemistry in Class 11 can be readily attained with the right guidance and notes.
Class 11 Chemistry has several significant and simple chapters that can readily assist students in achieving high exam scores. Chapter 4 Chemical Bonding and Molecular Structures is one such chapter. The Chapter 4 Chemistry Class 11 revision notes cover all the subjects, concepts, and phrases that are required for students to comprehend in a pleasant and straightforward manner, which will help them score well on exams.CBSE Class 11 Chemistry Notes Chapter 4 PDF
Chemical bonding and molecular structure notes from Class 11 Chemistry Chapter 4 become crucial during the test. Several knowledgeable Chemistry teachers have prepared these notes. Students may easily learn all the important information in the chapter by using the chemical bonding Class 11 notes. For students who are sincere about getting top scores, revision notes become essential.CBSE Class 11 Chemistry Notes Chapter 4 PDF
CBSE Class 11 Chemistry Notes Chapter 4
Introduction
Atoms are usually not capable of free existence but groups of atoms of the same or different elements exist as one species, e.g., H 2 O 2 , P 4 S s , H 2 O H2O2,P4 Ss,H2O . A molecule is a collection of atoms that exist as a single species and share common features. Clearly, some force is at work within the molecules to keep these atoms together.Chemical Bond
The process of creating a chemical bond between two or more atoms, molecules, or ions results in the formation of a chemical compound. Chemical bonds hold the atoms in the resultant molecule together.Types of Chemical Bonds Include
-
Ionic Bonds
-
Covalent Bonds
-
Hydrogen Bonds
-
Polar Bonds
Modes of Chemical Composition
when an atom to another totally transfers one or more electrons. This process is called electrovalency, and the chemical bond that results is referred to as an ionic or electrovalent bond.Through the sharing of electrons. This can happen in one of two ways:
- When the two joining atoms contribute the same number of shared electrons, a covalent connection is formed.
- When these electrons are entirely given by one atom yet shared by both, a coordinate bond, often referred to as a dative bond, is created.
Lewis Symbols
The idea of an electrical arrangement of noble gases was the basis for Kossel and Lewis's successful 1916 explanation of why atoms combine to form molecules. The tendency of noble gas atoms to combine with other noble gas atoms or atoms of other elements is negligible or nonexistent. This suggests that these atoms' electronic configurations have to be stable. Lewis represented the electrons in an atom's valence shell using straightforward symbols. The electrons in the outer shell are shown as dots surrounding the symbol for the atom. These symbols are called Lewis symbols or electron dot symbols.Ionic Bond
A type of chemical bonding known as ionic bonding is created when electrons are moved from one atom or molecule to another. In the process, one electron is lost by one atom and gained by another. One of the atoms gets a negative charge and is called an anion when an electron transfer takes place. The other atom that receives a positive charge is known as the cation. The stronger the ionic bond, the greater the charge differential between the anion and the cation. This is because the ionic bond is formed by the difference in charge between the two atoms.
Electrovalency
The number of electrons lost or acquired during the formation of an electrovalent connection is known as the element's electrovalency. For instance, the valencies of calcium and sodium were two and one, respectively, after they had lost one electron. With an electrovalency of one and two, respectively, chlorine and oxygen gain one and two electrons. Put differently, valency and ion charge are synonymous.Covalent Bond
Covalent bonding can be polar or nonpolar in nature. Because the more electronegative atom draws the electron pair away from the less electronegative atom and closer to itself, the electrons are shared unequally in polar covalent chemical bonds. One such molecule is water, which is polar. varying sections of the atom have varying charges due to the uneven electron spacing between them. One end of the molecule has a partly positive charge, and the other has a partial negative charge.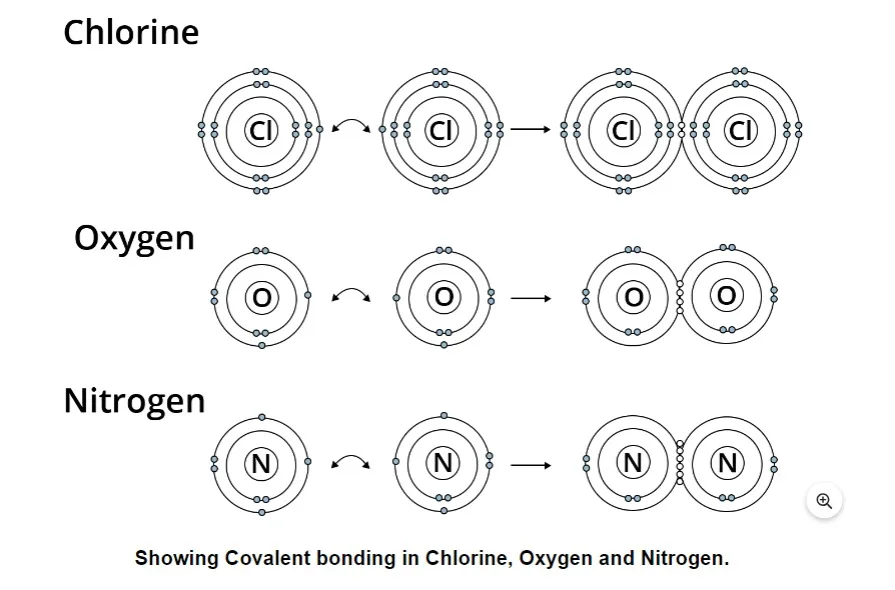
Hybridisation
The process of combining atomic orbitals that are part of the same atom but have slightly different energies, known as hybridization, produces new orbitals with the same energies and geometries as well as an energy redistribution amongst the original orbitals. The new orbitals that emerge from this process are called hybrid orbitals.Valence Bond Theory
Types of Covalent Bond
-
Sigma (σ) Bond.
-
Pi (π) Bond.
1. Sigma (σ) Bond:
This kind of covalent bond is created by the direct positive (same phase) overlap of atomic orbitals along the internuclear axis. Sigma bonds are the strongest covalent connections because the participating orbitals directly overlap each other. Electrons are defined as the electrons that are involved in a bond. Generally speaking, all single bonds are sigma bonds. They can be made using the following combinations of atomic orbitals.S-S Overlapping
In this kind of overlapping, one "s" orbital from each participating atom experiences head-on overlapping along the internuclear axis. An orbital must be half-filled before it can overlap with another orbital.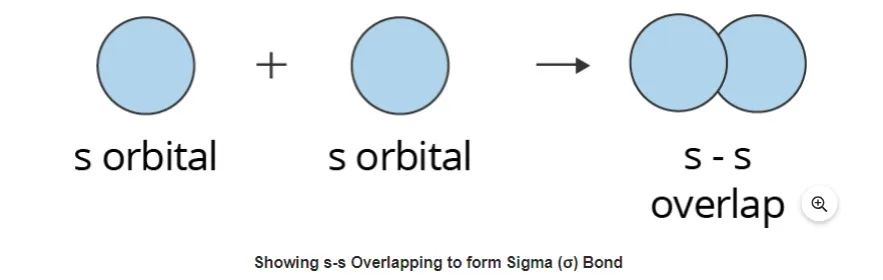
P-P overlapping
Each participating atom experiences a head-on overlapping of one half-filled p orbital along the internuclear axis under this situation. An example of this kind of overlapping is provided below.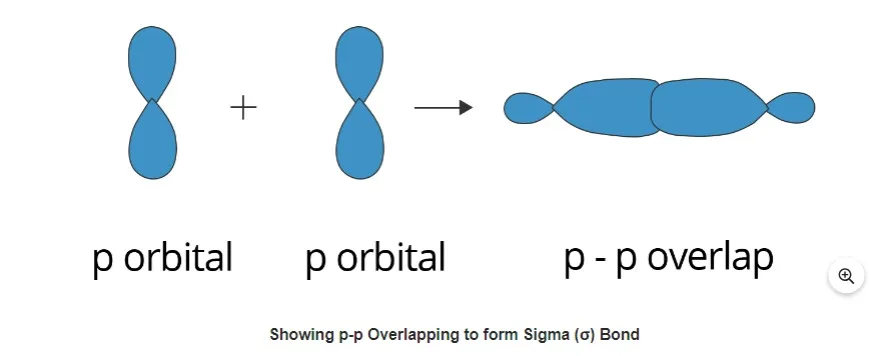
Dipole Moment
In chemistry, dipole moments convey a molecule's polarity or nature. The word dipole moment is defined as the product of the charge and the distance between the atoms in a chemical connection. Dipole moment (μ) of polar molecule = q×I if +q amount of positive charge is separated from -q amount of negative charge by bond distance I. The dipole moment of perfectly non-polar molecules such as nitrogen, oxygen, and hydrogen is zero, while that of polar molecules such as water, ammonia, and methane is positive. It is a tool for figuring out the residual charge, electric polarisation, and proportion of ionic character on the atoms in molecules.Benefits of CBSE Class 11 Chemistry Notes Chapter 4
Associated with the Most Recent Exam Patterns and Syllabus: Students can concentrate on the most pertinent subjects and formats that they are likely to encounter in their Class 12 Chemistry exams by using our fundamental subjective questions and notes, which are made to match the most recent curriculum and exam patterns.
Topic Coverage: The class 12 Chemistry notes include a comprehensive coverage of a wide range of topics that enable students to fully practice and comprehend different concepts within the curriculum. Building a solid foundation in the subject topic is aided by this discussion of fundamental subjective questions.
Comprehensive and Simple to Understand Solutions: We offers comprehensive answers, which help students better understand difficult concepts and grow from their errors.
CBSE Class 11 Chemistry Notes Chapter 4 FAQs
What is the important topic of Chapter 4 chemistry Class 11?
What are the 4 types of bonds in chemistry?
Which chapter is most important in chemistry class 11?










