CBSE Class 11 Chemistry Notes Chapter 13: Our website offers free downloads of the CBSE revision notes for class 11 Chemistry Chapter 13 hydrocarbons, making studying more enjoyable and simpler. Additionally, students can use these PDF notes, which include thorough explanations of all the significant chapter subjects. Students who use these PDF notes will be able to comprehend every subject matter with clarity and perform very well on their tests.
In the context of chemistry, hydrocarbons are defined as organic molecules made up of carbon and hydrogen atoms. However, there are a lot of topics we need to learn about when we study this topic in class 11, which is covered in the CBSE books of chapter 13. These include the classification of hydrocarbons, alkenes, alkanes, alkynes, toxicity, and carcinogenicity. We also need to understand equations and solve various problems.CBSE Class 11 Chemistry Notes Chapter 13 Overview
Hydrocarbons are chemicals that simply contain carbon and hydrogen. They come from the main energy sources, such as coal and petroleum. The main energy sources for the automotive industry are liquefied petroleum gas (LPG) and compressed natural gas (CNG), whereas domestic gasoline is derived from petroleum. In this chapter students will learn all about hydrocarbons. their tpyes and properties.- Some basic concepts of Chemistry
- Classification of the Elements & Periodicity in Properties
- Structure of Atom
- States of Matter - Liquids and Gases
- Chemical Bonding & Molecular Structure
- Equilibrium
- Thermodynamics
- Redox Reactions
- Hydrocarbons
- Environmental Chemistry
CBSE Class 11 Chemistry Notes Chapter 13 PDF
Notes from NCERT Class 11 Chemistry Chapter 13 are based on identifying the IUPAC nomenclature, structures, and methods of preparation of hydrocarbons. Both the application of hydrocarbons and the conformations of ethane are distinguished in the chapter Hydrocarbons Class 11 notes. The chemistry class 11 notes for Ch. 13 make it simpler to predict the additional, asymmetrical products of alkenes and alkynes. Here we have provided CBSE Class 11 Chemistry Notes Chapter 13 PDF for the ease of students.CBSE Class 11 Chemistry Notes Chapter 13 PDF
CBSE Class 11 Chemistry Notes Chapter 13
Classification Of Hydrocarbons
Based on the structure, hydrocarbons can be classified into four types:- Alkanes that are open-chain saturated.
- Unsaturated: Hydrocarbons with two or three covalent bonds connecting the carbon atoms to one another and a propensity to absorb additional hydrogen atoms are known as unsaturated hydrocarbons.
- Alkynes and alkenes, for instance.
- Alicyclic – cyclic
- Aromatic: These hydrocarbons are classified as cyclic hydrocarbons that resemble the benzene ring in nature and have alternating C-C and C=C bonds.
ALKANES
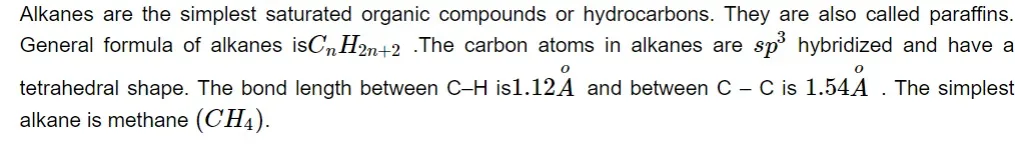
-
Newman Projection
2. SawHorse projection
Methods of Preparation
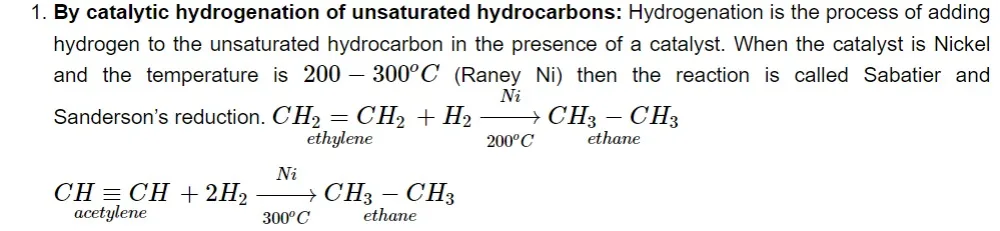 Instead of employing nickel as a catalyst, platinum or palladium is used to carry out hydrogenation at room temperature. This process is unable to make methane because an unsaturated hydrocarbon is devoid of all carbon atoms.
Instead of employing nickel as a catalyst, platinum or palladium is used to carry out hydrogenation at room temperature. This process is unable to make methane because an unsaturated hydrocarbon is devoid of all carbon atoms.

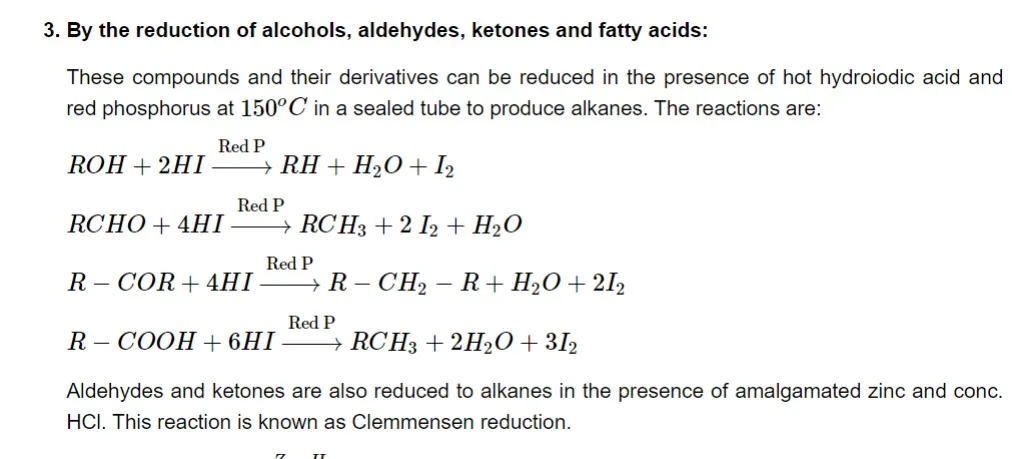
-
By condensing two molecules of alkyl halides:
When two molecules of alkyl halides are treated with sodium metal in the presence of dry ether, then they are coupled to form an alkane. This reaction is known as Wurtz synthesis.
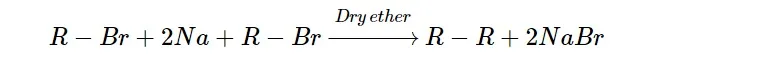
-
By decarboxylation of carboxylic acid:
when the sodium salt of a carboxylic acid is strongly heated with soda lime, then an alkane is formed by the elimination of C O 2 𝐶𝑂2 as carbonate.
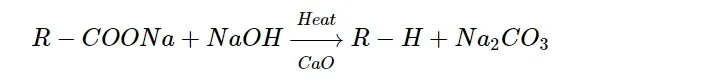
-
Kolbe’s electrolysis: This involves sodium or potassium salts of fatty acids to be electrolyzed that form higher alkanes at the anode.
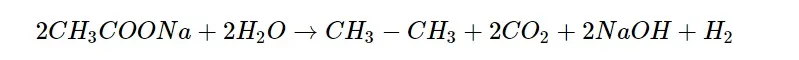
Physical Properties
As a result of their weak force composition, alkanes containing up to four carbon atoms are colourless and odourless gases, while the remaining thirteen constituents are colourless and odourless liquids. Alkanes are colourless, odourless solids starting at carbon-18. All alkenes, except ethene, have no smell and behave similarly to alkanes. The smell of ethene is nice. They are all devoid of colour. Alkynes and alkanes exhibit a similar trend.Density: As molecular mass rises, alkane density rises gradually until it reaches a constant value of 0.8.
Solubility: They are soluble in non-polar solvents like ether, carbon tetrachloride (CCl4), benzene, etc., but insoluble in polar solvents like water.
Melting and boiling points: The melting point of straight-chain alkanes rises in proportion to the number of carbons in the molecule. However, the melting point increases unevenly as molecule mass increases. In homologous series, alkenes and alkynes likewise exhibit a progressive rise in melting and boiling temperatures as molecular mass increases.
Their melting and boiling points are greater than those of equivalent alkanes, indicating that they are less volatile than alkanes.Chemical Properties
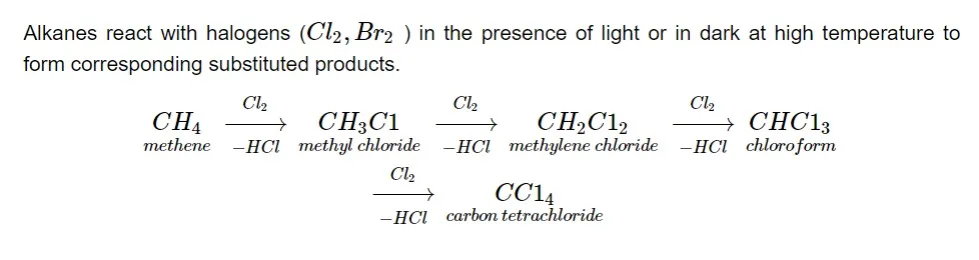
Because they contain non-polar C–C and C–H bonds, alkanes are extremely stable and inert molecules. Alkanes are saturated molecules with robust sigma bonds that remain intact in everyday situations. As a result, alkanes use a free radical mechanism to react at high temperatures.1. Halogenation (free radical substitution):
2. Nitration:
The nitro group is introduced during nitration. Alkanes with three or more carbon atoms may exist. A multitude of nitro compounds are produced when propane is nitrated.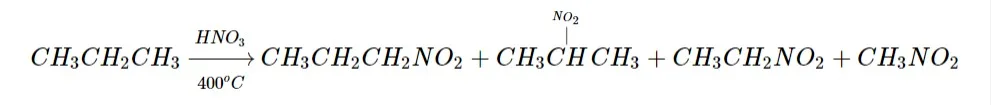
 Markonikov's rule governs addition in the situation of asymmetrical alkenes. Ionic mechanisms are involved in this process. The more stable carbocation is formed as an intermediate after electrophilic addition to a carbon–carbon double bond.
Markonikov's rule governs addition in the situation of asymmetrical alkenes. Ionic mechanisms are involved in this process. The more stable carbocation is formed as an intermediate after electrophilic addition to a carbon–carbon double bond.
ALKENES

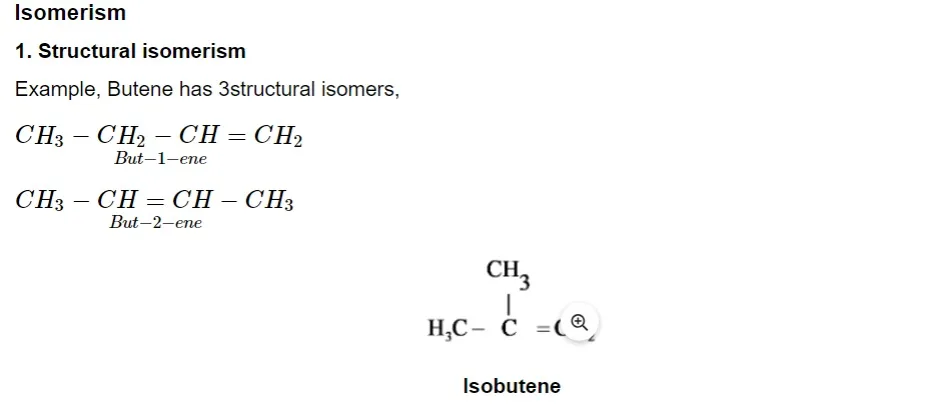
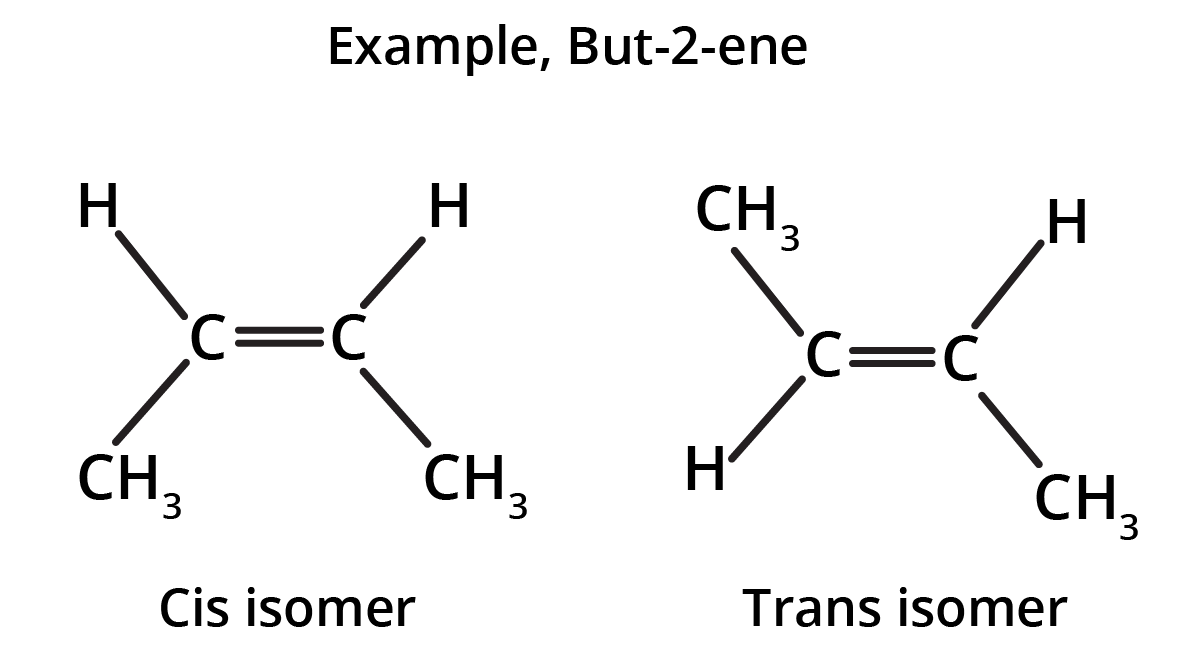
Geometrical isomers
The hindered rotation around the C – C bond gives rise to stereoisomers having different spatial arrangements. Two isomers exist as follows:
Methods of Preparation
1. By dehydration of alcohol: Dehydration of alcohol in the presence of acids forms alkene. The reaction is an elimination reaction.
2. By the dehydrohalogenation of alkyl halides: it involves an alkyl halide in the presence of alcoholic KOH to yield alkene.
If dehydrogenation of alkyl halide gives two products, the major product will be according to Saytzeff’s rule which states that the most substituted alkene will be the major product.
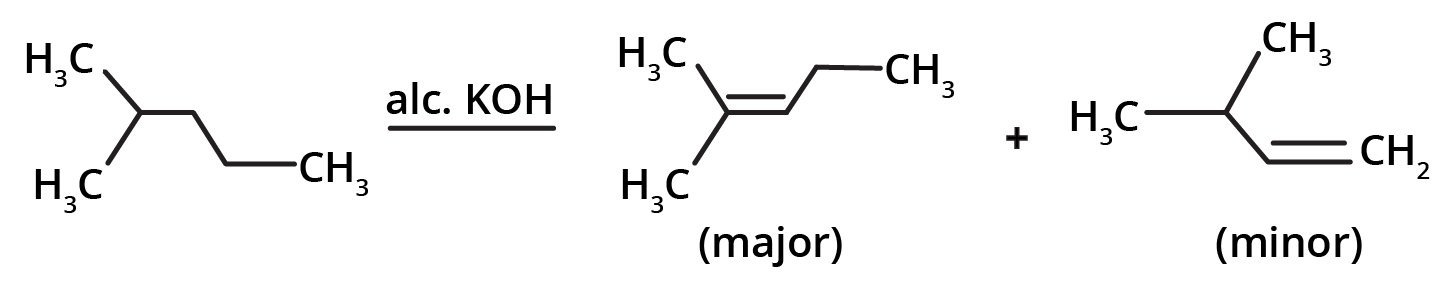
The ease of dehydrohalogenation has the order:
Tertiary alkyl halide > secondary alkyl halide > primary alkyl halide.
Alkyl halides follow the order:
alkyl iodide > alkyl bromide > alkyl chloride.
3. By the dehalogenation of vicinal dihalides: Dehydrohalogenation of vicinal dihalides in the presence of Zinc dust along with alcoholic solution yields pure alkene.
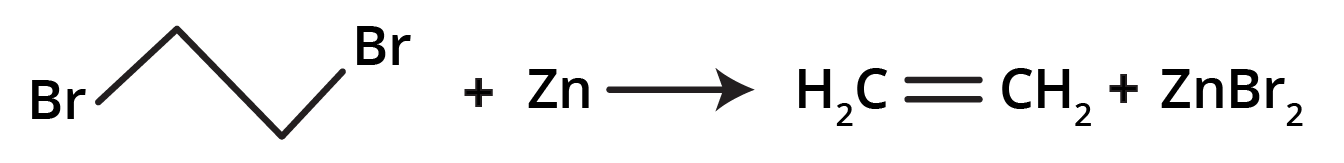
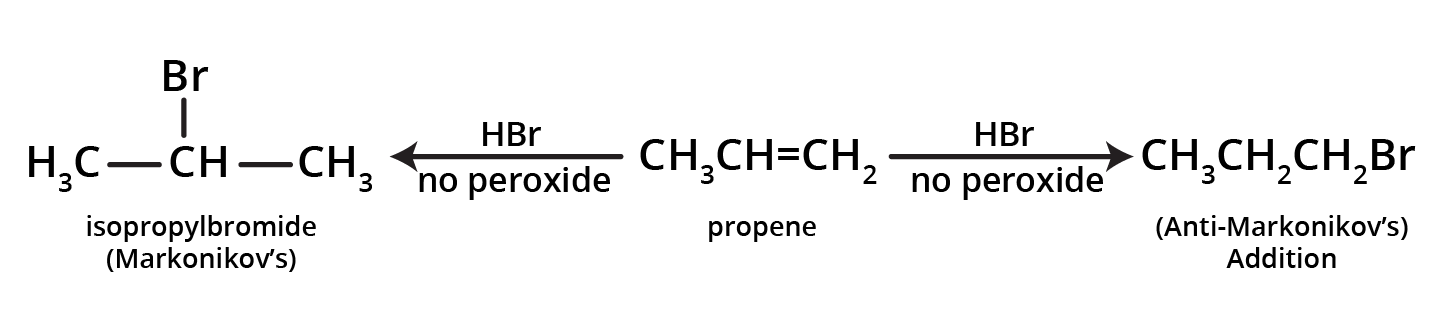
Deviation from Markonikov’s rule:
It has been observed that the addition of HBr to unsymmetrical alkenes like propene in the presence of air, peroxide or light yields n-propyl bromide by anti-Markovnikov's rule. The effect is also called the peroxide effect or Kharasch effect.
-
Addition of hypochlorous acid
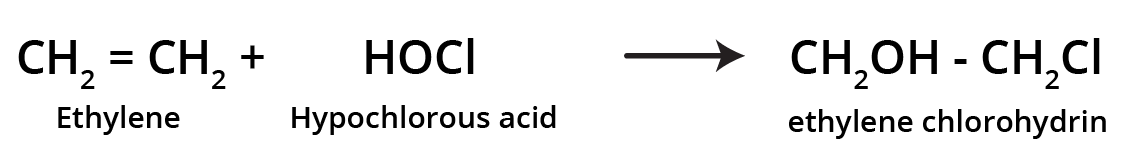
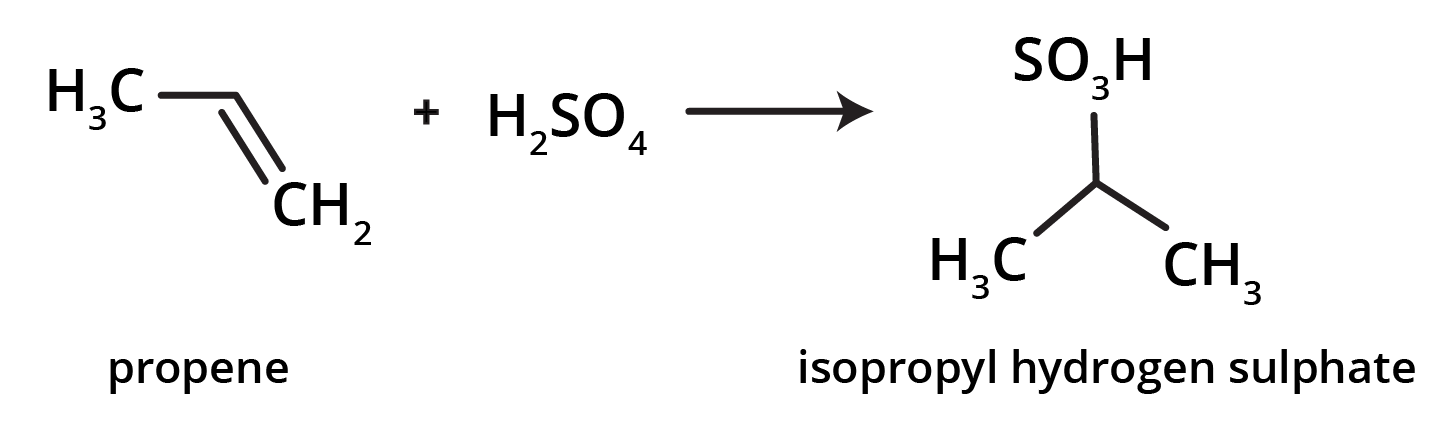
-
Addition of sulphuric acid
 3.
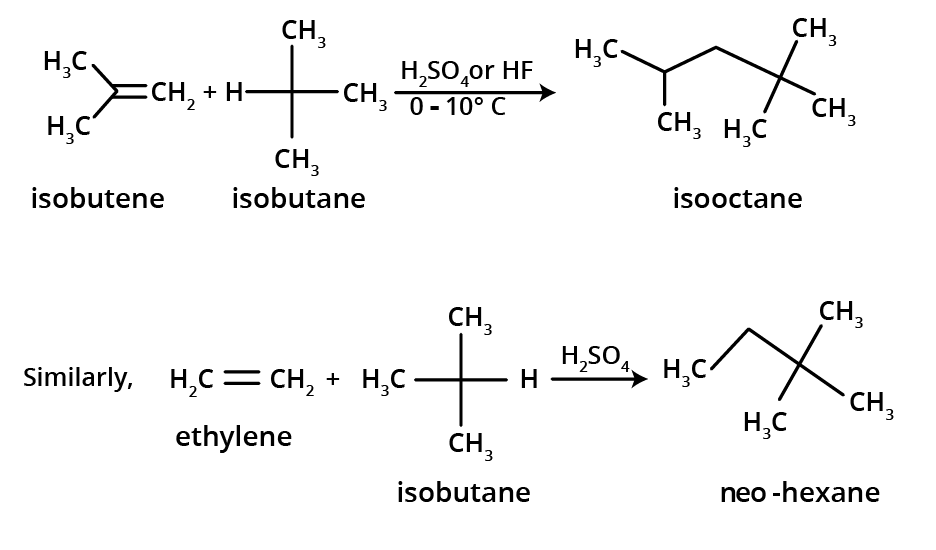
Addition of alkanes (alkylation)
3.
Addition of alkanes (alkylation)
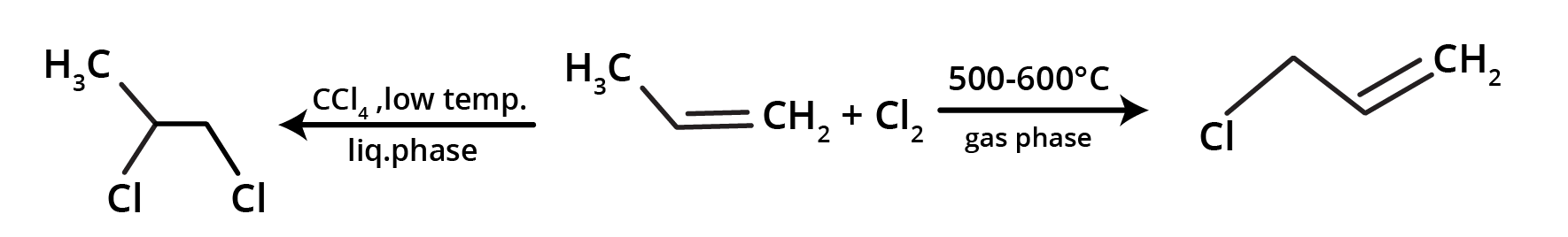
Substitution reaction:
Chlorination is done by treating the alkene with carbon tetrachloride in liquid phase or with chlorine gas:
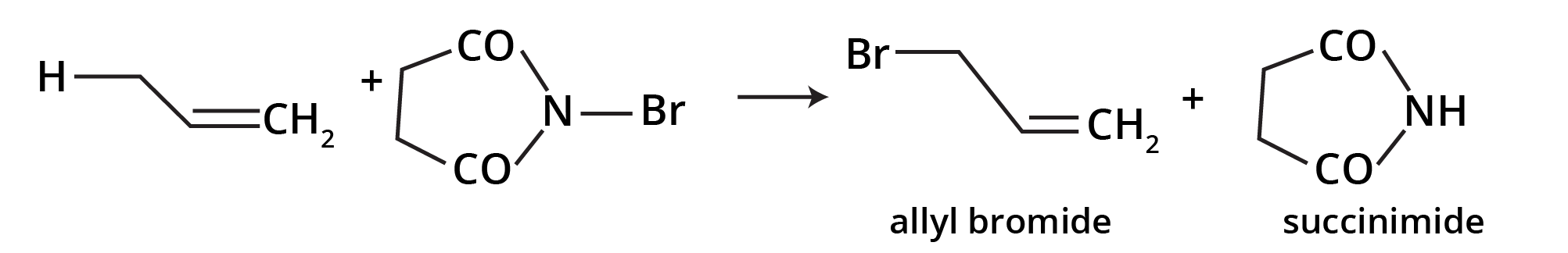
Allylic bromination (bromination at allylic carbon atom) is very easily achieved by treating the alkene having hydrogen atom at the allylic carbon atom with N-bromosuccinimde (NBS).
Chemical Properties
Because their electrons are loosely bonded, alkynes produce electrophilic addition reactions; nevertheless, these reactions happen more slowly in alkynes than in alkenes. Alkynes contain terminal hydrogen, which has an acidic character. The electrons in the bond with more s character will be closer to the nucleus because s electrons are closer to the nucleus than p electrons. The following list represents the quantity of s character in different types of C-H bonds: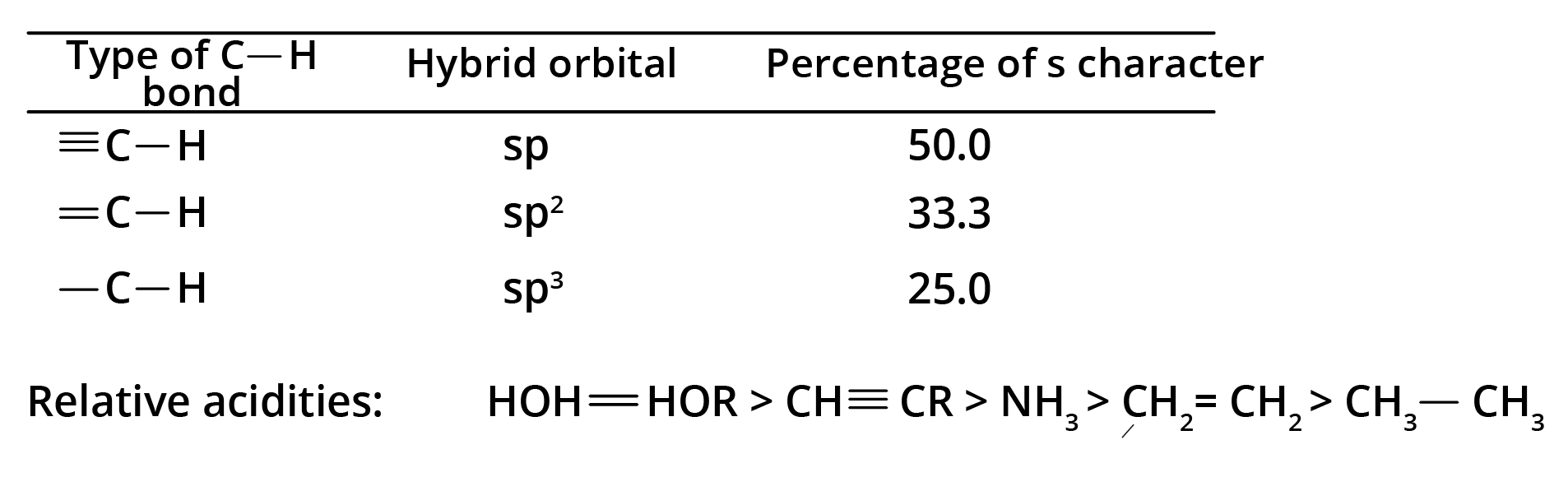
Benefits of CBSE Class 11 Chemistry Notes Chapter 13
During test days, students can review the complete syllabus with the aid of the Revision Notes of Class 11 Hydrocarbons Chemistry or the CBSE Class 11 Quick Revision Notes for Chemistry or other topics. All of the significant equations and ideas presented in the chapter are covered in these Hydrocarbons revision notes. Quick revision notes are helpful even for those who just want a summary of a chapter. Moreover, these notes will help you save time on hectic test days.CBSE Class 11 Chemistry Notes Chapter 13 FAQs
Which is the most important chapter in chemistry class 11?
Is Class 11 chemistry tough?
Which chapters are most scoring in chemistry class 11?