

CBSE Class 11 Chemistry Notes Chapter 11: The CBSE Class 11 Chemistry Notes Chapter 11 are free to download on our website. The p-Block Elements CBSE Class 11 Chemistry Notes Chapter 11 are now available chapter-by-chapter here, the greatest online learning resource for CBSE students, to help them quickly prepare for their school-based yearly exams and CBSE exams. You may also obtain the Class 11, Chapter 11.
CBSE Class 11 Chemistry Notes Chapter 11 Overview
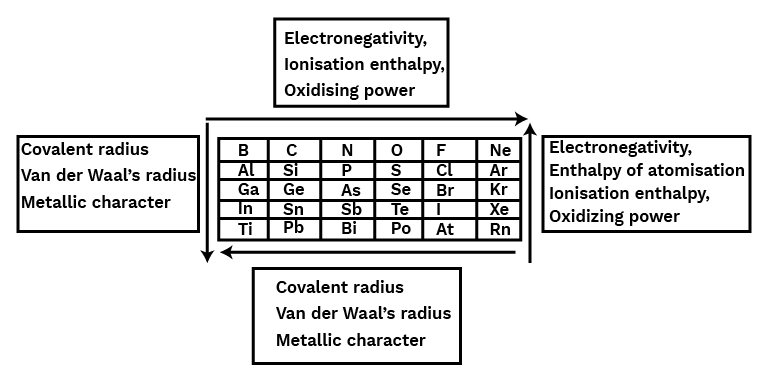
Six groups of p-block elements, numbered 13 through 18, with the electrical configuration ns2np1-6, are included in the periodic table. It contains every kind of element, including metal, non-metal, and metalloids. The differences in their electronic configuration's inner core have a significant impact on their chemical and physical properties. The group's lighter elements have a very consistent group oxidation state. The heavier elements have more stable lower oxidation states and produce dπ–dπ or dπ–pπ bonds. The lighter components, however, form pπ–pπ bonds. Second-period elements have a maximum covalence of 4, however heavier elements can have a greater value due to the lack of a d orbital.CBSE Class 11 Chemistry Notes Chapter 11 PDF
The CBSE Class 11 Chemistry Notes Chapter 11 are available for download, and they will help students do well on tests. CBSE Class 11 Chemistry Notes Chapter 11 how a group of knowledgeable professors prepares chemistry in class 11. You may quickly revise the entire chapter with the aid of the revision notes. One of the best study techniques that lecturers recommend is going over your CBSE Class 11 Chemistry Notes Chapter 11 again on exam day.CBSE Class 11 Chemistry Notes Chapter 11 PDF
CBSE Class 11 Chemistry Notes Chapter 11
The elements in groups 13 through 18 of the periodic table comprise the p-block. The p-block contains metals, metalloids, and non-metals. The p-block components have an electrical configuration similar to a general ns2np1−6 valence shell structure. The first members of each group from 13 to 17 of the p-block elements are distinct from the other members of their respective groups in numerous respects due to their small size, strong electronegativity, and lack of d-orbitals. In comparison to other members of the same group, the first member of a group is better able to form pπ−pπ multiple connections both to elements in the second row (e.g., C=O,C=N,C≡N,N=O) and to itself (e.g., C=C,C≡ C,N≡N). A p-block element's maximum oxidation is its group number minus 10. In groups 13 to 16, the oxidation state two less than the maximal group oxidation state becomes more stable because of the inert pair effect. (The reluctance of s-subshell electrons to form chemical bonds).Trends in Properties of P-Block Elements
(A) Group 13 Elements: The Boron Family
Aluminium is a metal that shares many chemical properties with boron, while gallium, indium, and thallium are almost fully metallic. Boron is a non-metal.Electronic Configuration:
The valence shell electronic configuration of elements of boron family is n s 2 n p 1 ns2np1 .
Atomic Radii:
The atomic radius increases as one advances along the group because each consecutive member gains an extra electron shell. Compared to Al, Ga has a smaller atomic radius. An additional 10 d-electrons only slightly reduces the outer electrons' screening effect due to gallium's larger nuclear charge. Consequently, the atomic radius of gallium (135 pm) is smaller than that of aluminium (143 pm).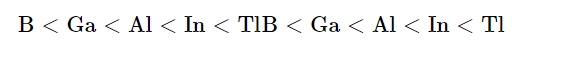
Ionization Enthalpy
The general patterns predict that the ionisation enthalpy values will drop smoothly along the group, however this is not the case. The decrease from B to Al corresponds with size increases. The discontinuity in ionisation enthalpy values between Al and Ga and between In and Tl is caused by the d and f electrons' low screening effect, which makes them unable to offset the increase in nuclear charge. The first three ionisation enthalpies of each element add up to a very high value.Electronegativity
As you go down the group, electronegativity decreases from B to Al and then considerably increases. This is because the atomic sizes of the various elements varies.Physical Properties
The element boron is non-metallic. It is an extremely hard solid black hue. It is available in several allotropic forms. Boron's crystalline lattice strength gives it a very high melting point. The remaining group consists of soft metals with low melting points and strong electrical conductivity. Due to its low melting point (303K), gallium may be liquid in the summer. The high boiling point (2676K) of this substance makes it suitable for use in high temperature measurements. There is an increase in element density from boron to thallium.Chemical Properties
Because boron is so little, its first three ionisation enthalpies add up to a very high value. Because of this, it is unable to create +3 ions and is only able to produce covalent compounds. But when we move from B to Al, the sum of Al's first three ionisation enthalpies decreases substantially, enabling Al to form Al+3 ions. Conversely, because of insufficient shielding, effective nuclear charge confines ns electrons tightly down the group, reducing their ability to participate in bonding. Thus, the p-orbital electron may be the only electron involved in bonding. Actually, reports of +1 and +3 oxidation states have been found for Ga, In, and Tl.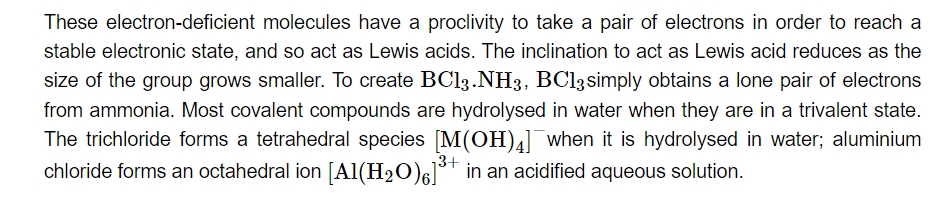
Reactivity Towards Air
 As you progress through the group, these oxides take on different characteristics. When boron trioxide, an acidic chemical, reacts with basic (metallic) oxides, it creates metal borates. Aluminium and gallium oxides are amphoteric, whereas indium and thallium oxides are basic.
As you progress through the group, these oxides take on different characteristics. When boron trioxide, an acidic chemical, reacts with basic (metallic) oxides, it creates metal borates. Aluminium and gallium oxides are amphoteric, whereas indium and thallium oxides are basic.
Reactivity Towards Acids and Alkalies
Although aluminium dissolves in mineral acids and aqueous alkalies, giving it an amphoteric quality, boron does not react with acids or alkalies, even at moderate temperatures. When aluminium is dissolved in diluted HCl, dihydrogen is produced. Conversely, concentrated nitric acid inactivates aluminium by covering the metal's surface with a layer of protective oxide. Dihydrogen is created when aluminium reacts with aqueous alkali.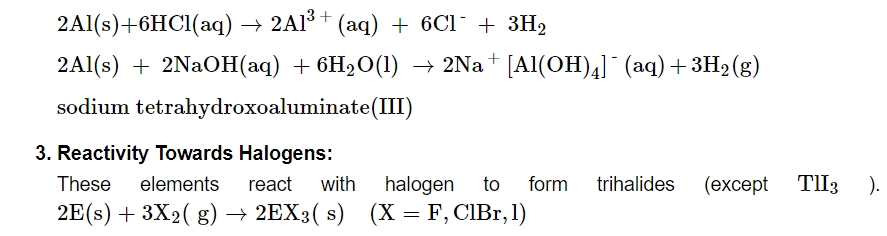
Boron (B)
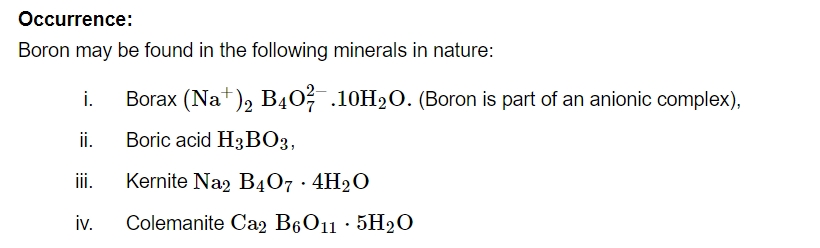

Uses:
Boron is utilised in the manufacture of high-impact steel and, since it absorbs neutrons, in reactor rods for atomic reaction control.
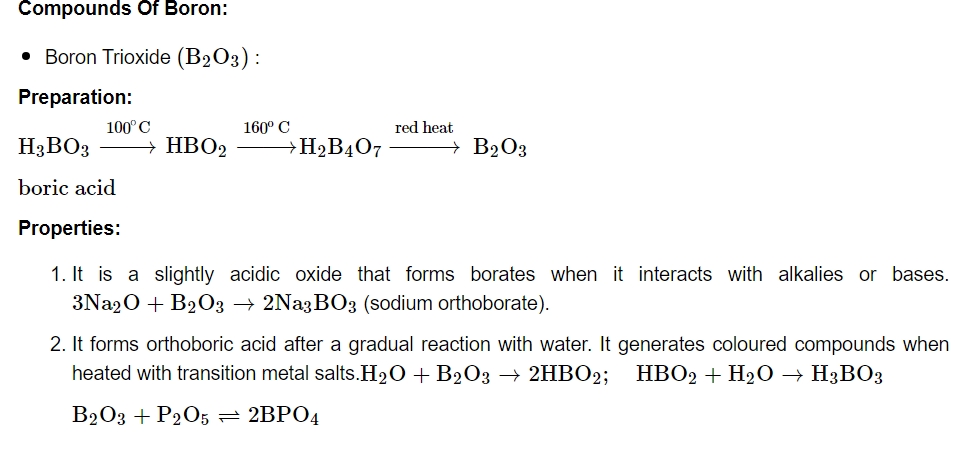
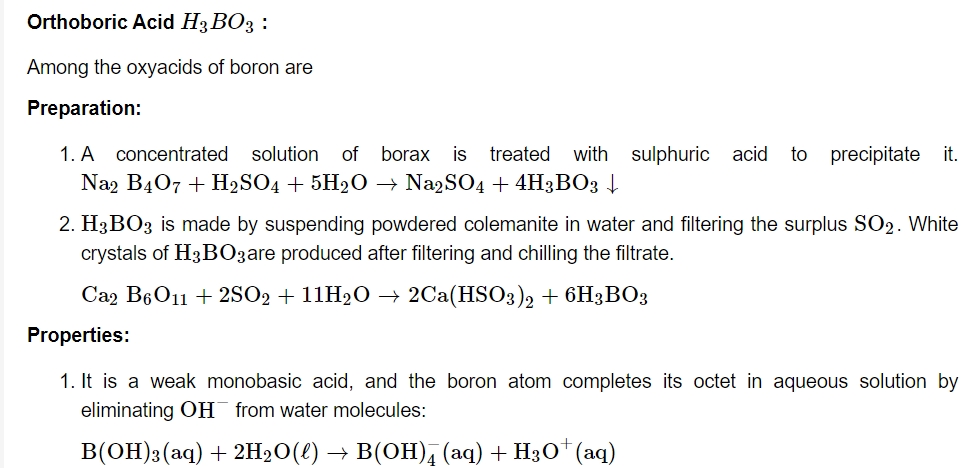
As a result, it acts as a Lewis acid rather than a proton donor. When a polyhydroxy molecule like glycol or glycerol is added to its aqueous solution, it behaves as a strong acid. The great stability of the conjugate bone chelate complex accounts for the acidity.
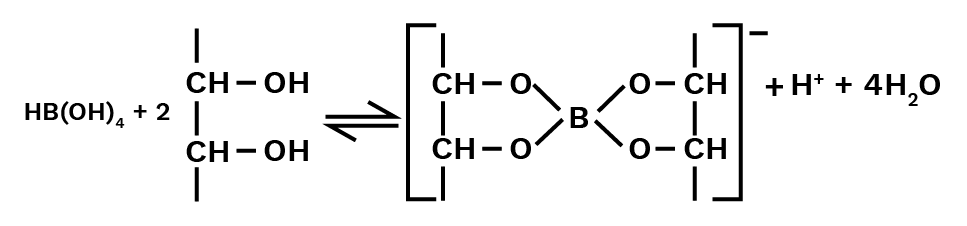
Catechol and salicylic acids create comparable complexes, but ethanol does not.
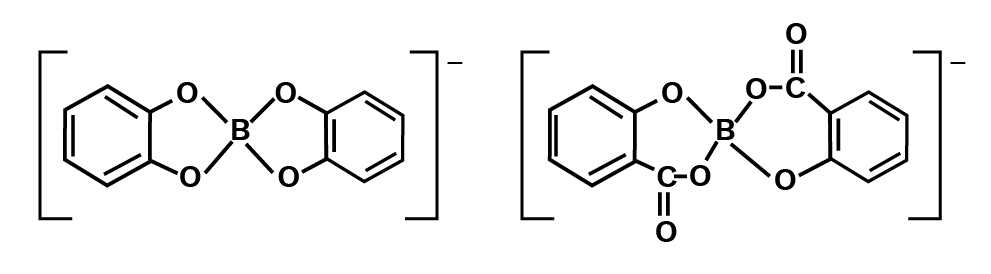
Orthoboric acid dissolves better in hot water than in cold, and it feels greasy to the touch. Planar units of BO3 are connected by hydrogen bonds to form a multilayer structure.
Uses:
It's an antiseptic, and the water solution may be used to cleanse your eyes. In addition, it is utilised in the glass, enamel, and pottery industries.
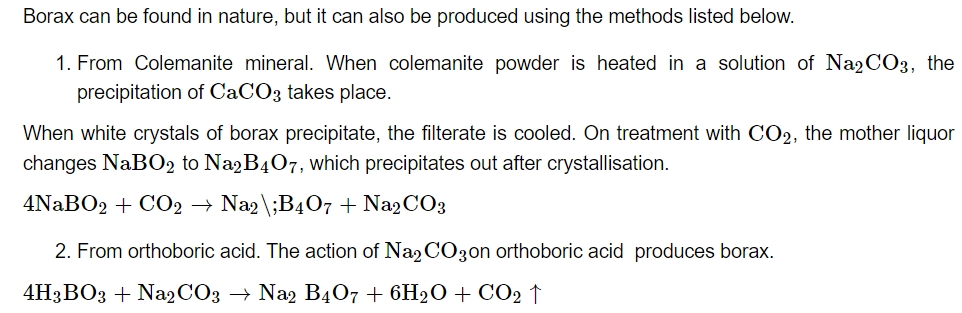
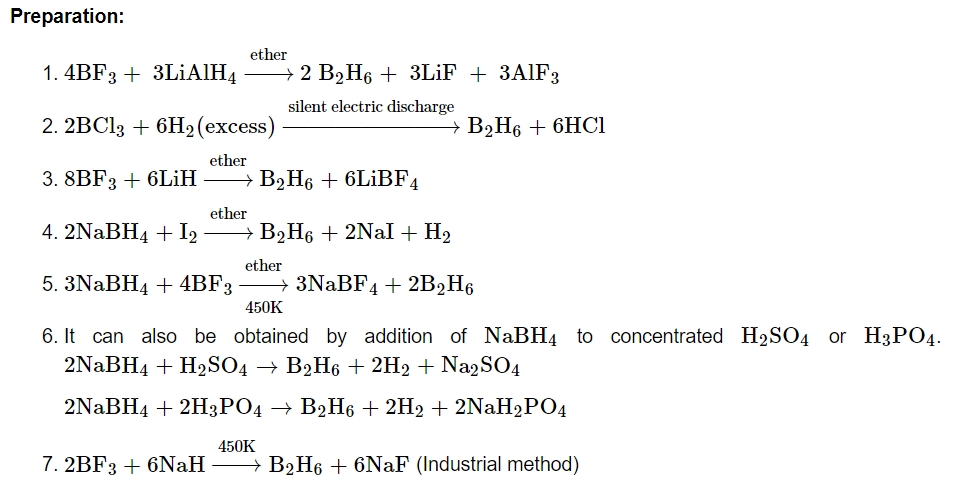
Preparation:
Aluminium
Extraction (Hall-Heroult Process)
Aluminium is extracted from the mineral ore known as bauxite (Al2O3⋅2H2O). To start, the ore is first purified using Bayere's technique. Al2O3 that is anhydrous is mixed and fused. Electrolytic reduction is applied to the fused combination of Na3AlF6 & CaF2 when aluminium is obtained at the cathode. Aluminium is purified by Hoope's technique.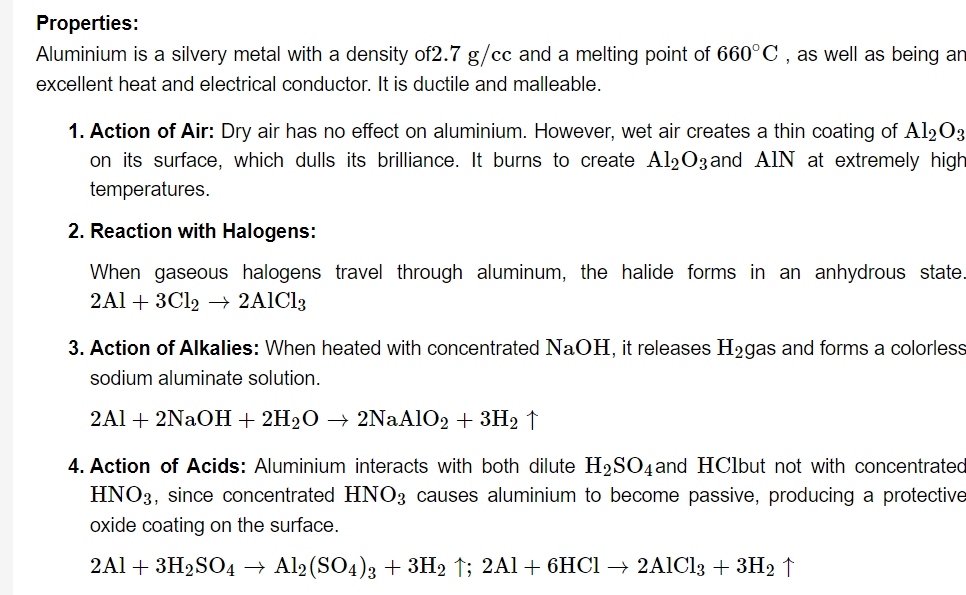
Uses
Aluminium is utilized for plating tanks, pipes, iron bars, and other steel items to prevent corrosion, manufacturing of aluminium cables, precise instruments, surgical apparatus, aircraft bodies, train coaches, motorboats, and automobiles.
Compounds of Aluminium
It's also known as alumina. It may be found in the form of bauxite and corundum in nature. It can also be found in gemstones. Topaz yellow, sapphire blue, ruby red, amethyst violet, and emerald green are some of the most important aluminium oxide stones.Properties:
It's a white amorphous powder that's insoluble in water but soluble in acids (forming, for example AlCl3 ) and alkalies, making it amphoteric. It's a polar covalent molecule with a positive charge.
Uses:
It is employed in the extraction of aluminium, the creation of fake gems, the production of aluminium compounds, and the fabrication of furnace linings. As a catalyst in organic processes, it is a refractory substance.
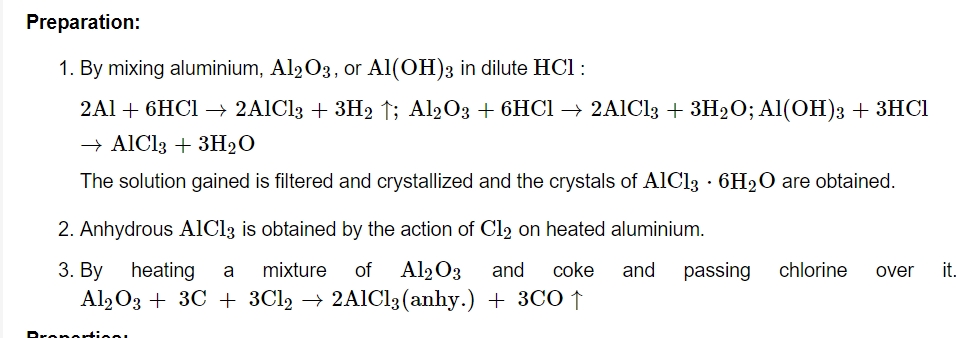
Oxidation States and Trends in Chemical Reactivity
Group 14 elements consist of four electrons in their outermost shell. The most common oxidation states for these elements are +4 and +2, along with carbon, which also exhibits negative oxidation states. Because the total of the ionisation enthalpies of the first four compounds is quite big, compounds at the +4 oxidation state are often covalent in nature. Higher in the sequence Ge<Sn<Pb, heavier components are more likely to exhibit the +2 oxidation state. It results from the valence shell electrons' ns2 incapacity to form bonds. As one moves through the group, the relative stability of these two oxidation states changes. Only four times may carbon surpass its covalence. It is something that other gang members are capable of. The reason for this is that they contain the d orbital. As a result, their halides are hydrolyzed, and they tend to accept electron pairs from donor species to form complexes.Allotropes of Carbon
There are several allotropic forms of carbon, including amorphous and crystallic forms. Graphite and diamond are two well-known crystalline forms of carbon. Fullerenes are a third type of carbon that were discovered in 1985 by H.W. Kroto, E. Smalley, and R.F. Curl.Diamond
It has a crystalline structure. Using hybridised orbitals, each carbon atom in a diamond is joined to four other carbon atoms in a tetrahedral pattern after undergoing sp hybridization. The C-C bond has a length of 154 pm. Expanding into space, the structure forms a rigid, three-dimensional network of carbon atoms. In this structure, directional covalent bonds are present throughout the lattice. Due to its remarkable resistance to breaking long-lasting covalent bonds, diamond is the hardest material in the universe. In addition to being used to create colours and tungsten filament for light bulbs, it is employed as an abrasive to sharpen hard tools.Graphite:
Graphite is a layered material. The layers are held together by van der Waal's forces, and there is 340 pm separating them. The carbon atoms in each layer are grouped into planar hexagonal rings. The C-C bond length in the layer is 141.5 pm. Every carbon atom in a hexagonal ring undergoes sp2 hybridization, resulting in the formation of three sigma bonds with neighbouring carbon atoms. And the fourth electron forms a π bond. The sheet is filled with scattered electrons. Graphite transfers electricity throughout the sheet because electrons are movable. Graphite cleaves easily between the layers, making it especially slick and smooth. Consequently, in machines that cannot utilise oil and must run at high temperatures, graphite is used as a dry lubricant.Fullerenes:
Graphite is heated in an electrical arc with inert gases like argon or helium to produce fullerenes. Fullerenes are the only pure form of carbon due to its smooth shape and absence of "dangling" connections. One type of molecule that resembles a cage is called a fullerene. The C60 compound known as buckminsterfullerene resembles a football. This set consists of twelve five-membered rings and twenty six-membered rings. A ring with six members can be fused with any other ring that has six members, whereas a ring with five members can only be fused with other rings that have six members. Every carbon atom is identical and experiences sp2 hybridization.Uses of Carbon
Plastic is mixed with graphite fibres to create composite materials that are lightweight and highly durable. Composites are used to make canoes, fishing rods, planes, and tennis rackets. Because graphite is a great conductor, it is utilised for industrial electrolysis and as electrodes in batteries. Acids and alkalies cannot be diluted using graphite crucibles. Activated charcoal is employed in air conditioning systems to control odour, water filters to eliminate organic pollutants, and hazardous gas absorbers. Carbon black finds application as a dark pigment in black ink and as a filler in automobile tyres. Coke is mostly used as fuel and as a reducing agent in metallurgy. A precious stone used in jewellery is the diamond.Benefits of CBSE Class 11 Chemistry Notes Chapter 11
- Additionally, all of these materials are available for download on our website. Students can use these files a day or two prior to the exam to help them prepare more effectively.
- All necessary notes are available for free of charge.
- Along with these, students can also receive the appropriate help with their syllabus, questions about any subject, and other related
CBSE Class 11 Chemistry Notes Chapter 11 FAQs
Which chapter has highest weightage in chemistry Class 11?
Is Class 11 chemistry difficult?
Which is the hardest chapter in Class 11 chemistry?












