
CBSE Class 12 Chemistry Notes Chapter 1: Class 12 Chemistry Notes for Chapter 1 The Solid State provides a detailed overview of the fundamental concepts related to solid-state chemistry. These notes cover important topics such as the classification of solids, including crystalline and amorphous solids, the arrangement of particles in different types of solids and the various crystal lattices and unit cells.
These notes are prepared by subject experts to ensure clarity and a deep understanding of the concepts making them an invaluable resource for students preparing for their board exams.CBSE Class 12 Chemistry Notes Chapter 1 The Solid State Overview
These notes are prepared by subject experts of Physics Wallah to provide a clear and concise overview of Chapter 1 The Solid State from Class 12 Chemistry. The notes cover key concepts such as the classification of solids, crystal lattices, unit cells, and the different types of packing in crystalline solids. With a focus on helping students grasp complex topics like voids, packing efficiency, and the calculation of density in solids, these notes are an important resource for thorough exam preparation.CBSE Class 12 Chemistry Notes Chapter 1 PDF
Here we have provided the PDF link for Class 12 Chemistry Notes Chapter 1 The Solid State. These notes are designed to help students grasp the essential concepts of the chapter, making it easier to study and revise. The PDF link is available below allowing you to download the notes and access them anytime, even without an internet connection. This resource is ideal for quick revisions and effective exam preparation.Class 12 Chemistry Notes Chapter 1 The Solid State PDF
CBSE Class 12 Chemistry Notes Chapter 1 The Solid State
Here we have provided CBSE Class 12 Chemistry Notes Chapter 1 The Solid State-Solids
Solids possess a definite volume, shape, and mass, which is attributed to the short distance between the particles and the strong interactions that hold them in fixed positions.Characteristic Properties of the Solid State
(i) Definite Mass, Volume, and Shape: Solids maintain a constant mass, occupy a specific volume, and have a defined shape. (ii) Short Intermolecular Distances: The particles within a solid are closely packed, resulting in minimal space between them. (iii) Strong Intermolecular Forces: The forces of attraction between particles in a solid are strong, keeping the particles tightly bound. (iv) Fixed Particle Positions: The constituent particles (atoms, molecules, or ions) are fixed in position and can only oscillate around their mean positions. (v) Incompressibility and Rigidity: Solids are generally incompressible and maintain their shape without deformation under normal conditions.Crystal Lattices and Unit Cells
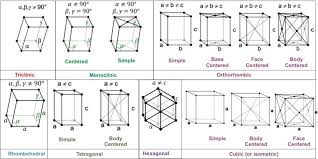
Unit Cell: The unit cell is the smallest repeating unit of a crystal lattice, serving as the fundamental building block of a crystal. By repeating the unit cell in three dimensions, the entire crystal structure is formed.
Types of Unit Cells: A crystal lattice can be generated by repeating a small portion known as the unit cell. There are several varieties of unit cells, each characterized by different arrangements of particles within the cell:
Primitive Cubic Unit Cell:
- Particles are located only at the corners of the cube.
Body-Centered Cubic Unit Cell:
- Particles are located at the corners and one in the center of the cube.
Face-Centered Cubic Unit Cell:
- Particles are located at the corners and at the centers of all the faces of the cube.
Crystal Lattices: A crystal lattice is a three-dimensional arrangement of points representing the positions of atoms, ions, or molecules in a crystal. It forms the framework upon which the crystal structure is built. A crystal lattice is made up of a repeating pattern of unit cells.
Characteristics of Crystal Lattice: (a) Each point in a lattice is referred to as a lattice point or lattice site.
(b) Each lattice point represents one constituent particle, which can be an atom, a molecule, or an ion. (c) Lattice points are connected by straight lines to illustrate the geometry of the lattice, revealing the overall symmetry and structure of the crystal.Number of Atoms in a Unit Cell
Primitive Cubic Unit Cell
The primitive cubic unit cell has atoms only at its corner. Each atom at a corner is shared between eight adjacent unit cells, four unit cells in the same layer, and four-unit cells in the upper or lower layer. Therefore, only 1/8th of an atom actually belongs to a particular unit cell.
Body-Centred Cubic Unit Cell
A body-centred cubic unit cell has an atom at each of its corners and also one atom at its body centre. Number of Atoms in BCC Cell: Thus, in a BCC cell, we have:- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 1 body center atom = 1 × 1 = 1 atom
Face-Centred Cubic Unit Cell
A face-centred cubic (FCC) unit cell is structured such that atoms are located at each of the eight corners of the cube and at the center of each of the six faces. The atoms at the face centers are shared between two adjacent unit cells, meaning that only half of each face-centered atom belongs to an individual unit cell.Number of atoms in BCC cell
a) 8 corners × 18 per corner atom = 8 × 18 = 1 atom b) 6 face-centered atoms × 12 atom per unit cell = 3 atoms Hence, the total number of atoms in a unit cell = 4 atoms Thus, in a face-centred cubic unit cell, we have:- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 6 face-centered atoms × 1/2 atom per unit cell = 3 atoms
Close-Packed Structures
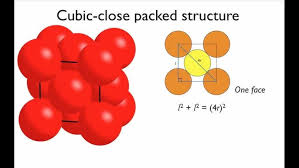 In solids, close packing refers to the arrangement of constituent particles in a manner that minimizes the vacant or empty space within the structure.
In solids, close packing refers to the arrangement of constituent particles in a manner that minimizes the vacant or empty space within the structure.
(a) Close Packing in One Dimension
In one-dimensional close packing, the arrangement of spheres (or atoms) is linear. Here’s how it works:- Arrangement: Spheres are aligned in a single row where each sphere is directly in contact with its neighboring spheres. This means that each sphere touches two other spheres, one on either side.
- Coordination Number: The coordination number in close packing is defined as the number of nearest neighbor particles that surround a given particle. In one-dimensional close packing, each particle (sphere) has exactly two nearest neighbors — one on the left and one on the right.
(b) Close Packing in Two Dimensions
In two-dimensional close packing, spheres are arranged in a way that maximizes the packing density and minimizes empty space within a plane. Here’s how this can be achieved:Close Packing in Two Dimensions
In two-dimensional close packing, spheres (or particles) are arranged in a planar pattern where they touch each other. This arrangement can be done in two main ways:Square Close Packing and Hexagonal Close Packing
Packing in Solids: One and Two Dimensions
Formula of a Compound and Number of Voids Filled
In a close-packed structure, such as in crystalline solids, voids or gaps occur between the constituent particles (atoms, ions, or molecules). These voids are crucial for understanding the packing efficiency and structural properties of the solid. There are two primary types of interstitial voids in a three-dimensional (3D) structure:Tetrahedral and Octahedral void
Packing Efficiency
Packing Efficiency is the percentage of total space filled by the particles.Packing Efficiency in hcp and ccp Structures
Hexagonal close packing (hcp) and cubic close packing (ccp) have the same packing efficiency.Packing Efficiency of a Unit Cell
Efficiency Packing in Body-Centred Cubic Structures
In a body-centred cubic unit cell, one atom is located at the body centre apart from the corners of the cube.Packing Efficiency in Simple Cubic Lattice
In the simple cubic unit cell, atoms are located at the corners of the cube.Calculations Involving Unit Cell Dimensions
The unit cell can be seen as a three-dimensional structure containing one or more atoms. We can determine the volume of this unit cell with the knowledge of the dimensions of the unit cell. Mass of unit cell = number of atoms in unit cell × mass of each atom = z × m Where, z = number of atoms in the unit cell, m = Mass of each atom The mass of an atom can be given with the help of Avogadro number and molar mass as: M/N A Where M = molar mass N A = Avogadro’s number The volume of the unit cell, V = a 3 => Density of unit cell = mass of unit cell/ volume of the unit cell => Density of unit cell = m/V = z×ma/a 3 = z×M/a 3 ×N AImperfections in Solids
Point Defects:
Point defects in solids are irregularities or deviations from the ideal arrangement of atoms or ions at a single point in the crystal lattice. These defects occur when the crystallization process is too rapid or when there are deviations in the regular pattern of particles. Point defects can significantly impact the properties of the material, such as its density, electrical conductivity, and mechanical strength. They are classified into three main types:Stoichiometric Defects:
Definition: In stoichiometric defects, the ratio of positive to negative ions in a solid remains unchanged, preserving the overall electrical neutrality of the compound. These defects do not disturb the stoichiometric ratio of the ions but affect the lattice structure.
Types:
- Vacancy Defect: This occurs when an atom or ion is missing from its regular lattice position, creating a vacancy in the crystal structure.
- Interstitial Defect: This defect arises when an extra atom or ion is present in the interstitial spaces (gaps) between the regular lattice points.
Frenkel Defect:
Definition: The Frenkel defect is commonly observed in ionic solids, where a smaller ion (usually a cation) moves from its normal lattice position to an interstitial position. This defect creates both a vacancy (where the ion was originally located) and an interstitial defect (where the ion now resides).
Characteristics: This type of defect maintains electrical neutrality, as the total number of positive and negative charges remains balanced.
Schottky Defect:
- Definition: Schottky defects occur in ionic solids when equal numbers of cations and anions are missing from the lattice, creating vacancies. This type of defect is crucial for maintaining electrical neutrality in the material.
- Characteristics: The loss of ions leads to a decrease in density, and this defect is more common in solids where the sizes of cations and anions are similar.
Electrical Properties
Solids can be classified into three types on the basis of their conductivities. They are: (i) Conductors (ii) Insulators (iii) SemiconductorsMagnetic Properties
Magnetic properties of materials are studied by placing them in a uniform magnetic field and observing their behavior as the magnetic field is varied. The response of a material to a magnetic field depends on its magnetic properties, which are categorized into five major types: (i) Diamagnetic materials (ii) Paramagnetic materials (iii) Ferromagnetic materials (iv) Antiferromagnetic materials (v) Ferrimagnetic materialsBenefits of CBSE Class 12 Chemistry Notes Chapter 1 The Solid State
- Clear Definitions: Provides precise definitions and explanations of concepts, aiding in better understanding and retention.
- Visual Aids: Includes diagrams and illustrations of unit cells, packing structures, and defects, which help visualize and remember complex structures.
- Quick Revision: Summarizes important points and formulas, making it convenient for quick revision before exams.
- Efficient Study: Organizes information in a structured manner enabling efficient study sessions and effective time management.
CBSE Class 12 Chemistry Notes Chapter 1 The Solid State FAQs
What are the main types of solids?
What is a unit cell?
What is the importance of close packing in solids?
What are the magnetic properties of materials?

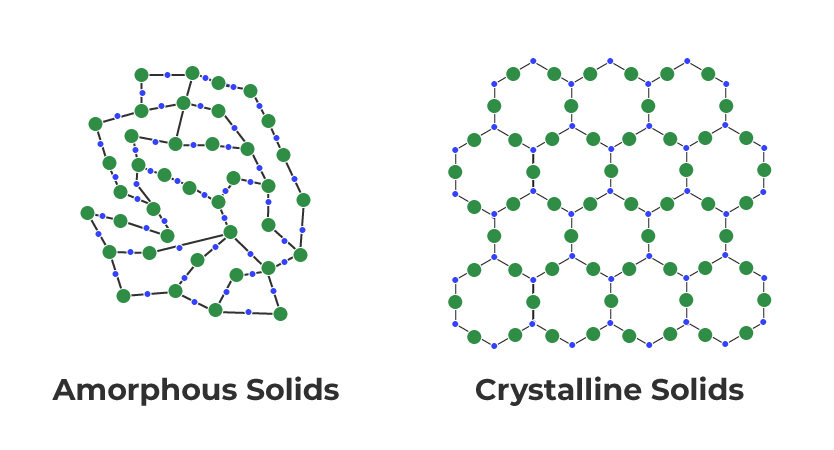 Solids can be classified into two types based on the order of particle arrangement:
crystalline
and
amorphous
solids.
Solids can be classified into two types based on the order of particle arrangement:
crystalline
and
amorphous
solids.
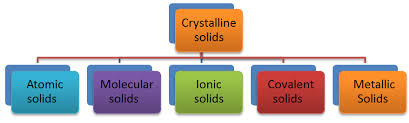 Crystalline solids are classified based on the nature of the interactions between their constituent particles. These interactions determine the properties of the solids, leading to the following four categories:
Crystalline solids are classified based on the nature of the interactions between their constituent particles. These interactions determine the properties of the solids, leading to the following four categories:









