
CBSE Class 12 Chemistry Notes Chapter 3: CBSE Class 12 Chemistry notes are important for students studying Chemistry, as Electrochemistry is a fundamental topic that requires a deep understanding of key concepts.
Our notes cover crucial aspects such as electrochemical cells, standard electrode potentials, and the Nernst equation. These concepts are pivotal in understanding how chemical energy is converted into electrical energy and vice versa. By using our notes students can enhance their comprehension of Electrochemistry and significantly improve their exam performance.CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry Overview
These notes on Electrochemistry for CBSE Class 12 Chemistry are prepared by the subject experts of Physics Wallah. They provide a detailed overview of key concepts such as electrochemical cells, electrode potentials, and the Nernst equation. The notes are designed to make complex ideas easier to understand, helping students study more effectively. With these notes students can better prepare for their exams and improve their understanding of Electrochemistry.CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry PDF
The PDF link for the CBSE Class 12 Chemistry Notes on Chapter 3 Electrochemistry is available below. You can download the PDF to access detailed notes and study materials that will help you grasp the essentials of Electrochemistry more effectively.CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry PDF
CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry
Here we have provided CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry-Introduction to Electrochemistry
Electrochemistry deals with generating electricity from chemical reactions and using electrical energy to drive chemical changes. This field has practical applications in various technologies, such as batteries, fuel cells, and electroplating. It is also used in the purification of metals and in creating energy-efficient and less polluting processes.Electrochemical Cells
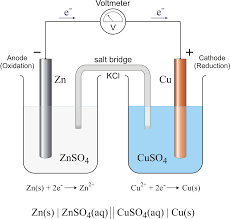 Electrochemical cells are devices that facilitate the conversion between chemical energy and electrical energy. They come in two main types:
Electrochemical cells are devices that facilitate the conversion between chemical energy and electrical energy. They come in two main types:
- Galvanic (Voltaic) Cells : These cells generate electric current from spontaneous chemical reactions. An example is the standard 1.5V battery used in everyday devices like remotes and toys.
- Electrolytic Cells : These cells use electrical energy to drive non-spontaneous chemical reactions.
- Cathode : The positive electrode where reduction (gain of electrons) occurs.
- Anode : The negative electrode where oxidation (loss of electrons) occurs.
Half Cells and Cell Potential
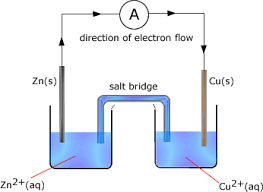 Electrochemical cells are made up of two half cells, each containing an electrode immersed in an electrolyte. A salt bridge connects these half cells, allowing ionic contact while preventing the mixing of solutions. The cell potential, or the voltage produced by the cell, is determined by the electrode potentials of the half cells. Electrode potentials are measured relative to a standard reference electrode, such as the standard hydrogen electrode.
Electrochemical cells are made up of two half cells, each containing an electrode immersed in an electrolyte. A salt bridge connects these half cells, allowing ionic contact while preventing the mixing of solutions. The cell potential, or the voltage produced by the cell, is determined by the electrode potentials of the half cells. Electrode potentials are measured relative to a standard reference electrode, such as the standard hydrogen electrode.
Galvanic Cells vs. Electrolytic Cells
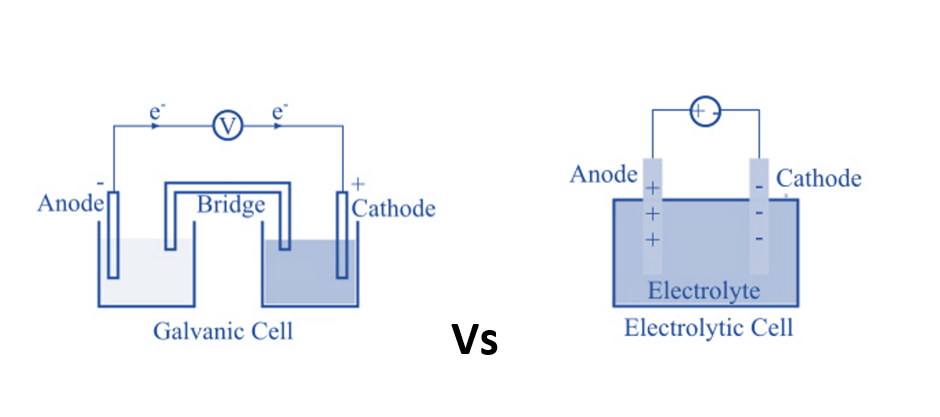
Galvanic Cells (Voltaic Cells):
Galvanic cells convert chemical energy into electrical energy through spontaneous reactions. In these cells, a chemical process occurs naturally, generating an electric current. The anode in a galvanic cell is negative, as it is the site of oxidation, while the cathode is positive, where reduction takes place. Electrons flow from the anode to the cathode in the external circuit. For instance, in a Daniell Cell, zinc (Zn) undergoes oxidation, and copper ions (Cu²⁺) undergo reduction:- Oxidation Half-Reaction (Anode): Zn(s) → Zn²⁺(aq) + 2e⁻
- Reduction Half-Reaction (Cathode): Cu²⁺(aq) + 2e⁻ → Cu(s)
Electrolytic Cells:
Electrolytic cells use electrical energy to drive non-spontaneous chemical reactions. In these cells, an external electrical source (like a battery) provides the energy required to induce the reactions. Electrolytic cells have a positive anode and a negative cathode, opposite to the polarities in galvanic cells. Ions in the electrolytic solution migrate towards the electrodes where they undergo reduction and oxidation. For example, in electrolysis of water, the electrodes are submerged in an electrolyte, and electrical energy is used to split water into hydrogen and oxygen gases.Measurement of Electrode Potential
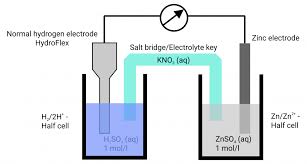 To measure the electrode potential, a standard hydrogen electrode (SHE) is used as a reference. The electrode in question is connected to the SHE, forming a complete electrochemical cell. The electrode at the negative terminal is assigned a negative potential, while the electrode at the positive terminal is assigned a positive potential. The potential difference between these two electrodes is measured with a potentiometer, and the direction of electric current flow in the external circuit is monitored using a galvanometer.
For instance, in the Daniell Cell:
To measure the electrode potential, a standard hydrogen electrode (SHE) is used as a reference. The electrode in question is connected to the SHE, forming a complete electrochemical cell. The electrode at the negative terminal is assigned a negative potential, while the electrode at the positive terminal is assigned a positive potential. The potential difference between these two electrodes is measured with a potentiometer, and the direction of electric current flow in the external circuit is monitored using a galvanometer.
For instance, in the Daniell Cell:
- At the anode, zinc undergoes oxidation: Zn → Zn²⁺ + 2e⁻
- At the cathode, copper ions undergo reduction: Cu²⁺ + 2e⁻ → Cu
Reference Electrode
Reference electrodes are used to measure the potential of other electrodes by providing a known, stable reference potential. There are two main types:Primary Reference Electrode : The Standard Hydrogen Electrode (SHE), also known as the Normal Hydrogen Electrode (NHE), is the most commonly used primary reference electrode. It consists of a platinum wire coated with finely divided platinum black. This wire is sealed into a glass tube and immersed in a beaker containing 1M hydrochloric acid (HCl) solution. Hydrogen gas is bubbled through the solution at a pressure of 1 atm and a temperature of 298 K (25°C). The SHE provides a reference potential of 0 volts under standard conditions.
Secondary Reference Electrode : An example is the Calomel Electrode. This type of reference electrode is used in conjunction with the SHE for various electrochemical measurements but is more practical for routine use due to its stable and reproducible potential.
Half cell is Pt H2(1 atm) H+(1M) Ecell = Ecathode – Eanode Ecell = Ecathode – 0 = Ecathode The measured Emf of the cell: Pt| H2 (g, 1 bar)| H+ (aq, 1M) || Cu2+ (aq, 1M)| Cu is 0.34 V. The positive significance of the standard electrode potential signifies the easy reduction of Cu2+ ions than H+ ions. The measured Emf of the cell Pt| H2 (1 bar)| H+ (1M) || Zn2+ (1M)| Zn is -0.76 V. The negative value of the standard electrode potential signifies that the H+ ions oxidise the Zn ions). Nernst Equation Under this section of Class 12 Chemistry Chapter 3 notes, students get a detailed explanation of the Nernst Equation. The Nernst equation is named by” German physicist Walther Nernst”. This equation guarantees cell potential under nonstandard conditions, relates the measured potential to the reaction quotient, and permits the exact measurement of equilibrium constants. Let us consider an electrochemical reaction shown in the following type aA + bB ⇾ cC + dD The equation can be written as follows. Ecell = E–cell – RT/ nF lnQ = E–cell – RT/ nF ln [C]c [D]d —————- [A]a [B]b E= emf of cell, n = Number of electron exchange, F = Faraday constant E –cell – RT/ 2F ln [Zn2+] —————- [Cu2+] In the case of Daniel’s cell, the Nernst equation is shown as follows. Ecell = E–cell – RT/ nF lnQ This equation implies that the value increases with the increase in the concentration of Copper ion and decreases the concentration of Zn ion. Substituting the values of R, F at T= 298 K. the equation becomes E –cell – 0.059/ 2 log [Zn2+] —————- [Cu2+] If the circuit in Daniel cell is closed: Zn(solid) + Cu2+ (aq) →Zn2+ (aq) + Cu(s) There is no change in the concentration of Cu2+ and Zn2+ ions after some time, and the voltmeter gives zero reading. At this point, equilibrium has been attained. So the Nernst equation may be written as: E = E –cell – 2.303 RT/ 2F log [Zn2+] —————- [Cu2+] Or E–cell = 2.303 RT/ 2F log [Zn2+] —————- [Cu2+] But at equilibrium, [Zn2+] —————-=Kc [Cu2+] and at T = 298 K. The above equation can be rewritten as E–cell = 0.059 V/2 log Kc {E–cell =1.1V} E–cell = log Kc =1.12 x 2 /0.059 V =37.28 Kc =2 x 1037 at 298 KElectrochemical cell and Gibbs Energy reaction
Electrical work done in 1 second equals electrical potential multiplied by the total charge passed. Passing the charges reversibly through the galvanic cell results in maximum work. Reversible work done using a galvanic cell is equivalent to a decrease in Gibbs energy; therefore, if the emf of the cell is E and nF is the amount of charge passed, and Δr G = Gibbs energy of the reaction, then. Δr G = -nFE cell For the reaction, E cell is an intensive parameter, but Gibbs energy is an extensive thermodynamic property, and the value depends on n, Thus if we write the reaction Zn(s) + Cu2+ (aq) –> Zn2+ (aq) + Cu(s) [Δr G = -2FE cell ] But when we write the equation 2Zn(s) + 2Cu2+ (aq) –> 2Zn2+ (aq) + 2Cu(s) [Δr G = -4FE cell ]Read More - 10 Things Every Class 12 Student Must Know for Board Exam Success
Electrolytic conductors (electrolysis)
Electrolysis is a process where electrical energy is used to drive a chemical reaction that wouldn't normally occur on its own. It involves the movement of ions toward oppositely charged electrodes, leading to the decomposition of the substance being electrolyzed. i) Ohm law: The current (I) carried by a conductor or electrolytic solution is directly proportional to the potential difference(V) between the two ends of the conductor. Vα I V = RI ii) Electrical Resistance: The electrical resistance is directly proportional to its length, Inversely proportional to its A, area of cross-section. It is denoted by the symbol ‘R’. It is measured in a SI unit called ohm (Ω) or volt/ ampere with the help of a Wheatstone bridge. R α I/A So R = ⲣ I/ A In the equation, the constant of proportionality denoted by rho is resistivity. It is measured in units called ohm metres.When length is 1m and cross-sectional area = 1m2 . Then resistivity becomes the resistance. 1 Ω m = 100 Ω cm 1 Ω cm = 0.01 Ω m iii) Conductance: It is the property of a conductor(metallic or electrolytic) that facilitates the flow of electricity. It is inversely proportional to resistance, and the si unit is ohm-1 or mho. G = I /R = A / ⲣ I iv) Specific conductance (conductivity): It is a reciprocal of specific resistance. ? = 1 / ⲣ = I /R .A = 1/R. I/A It is denoted by the symbol (Greek, kappa). It is measured in a unit called Sm–1. Then conductivity becomes the conductance. The conductivity of an electrolytic(ionic) solution depends on- The nature of the electrolyte added
- Size of the ions formed and their solvation
- The nature of the solvent as well as its viscosity
- The concentration of the electrolyte
- Temperature
Conductivity:
- Conductivity (κ) measures how well a solution conducts electricity. It depends on the concentration of the electrolyte.
- For both strong and weak electrolytes, conductivity decreases as the concentration of the solution decreases.
- This happens because, with dilution, the number of ions per unit volume decreases. Fewer ions mean less ability to carry an electric current.
Molar Conductivity:
- Molar conductivity (Λm) is the conductivity of a solution divided by the concentration of the electrolyte.
- Molar conductivity increases with dilution for both strong and weak electrolytes.
- As the concentration decreases, the number of ions per unit volume decreases, but the ions become more spread out and can move more freely, increasing the molar conductivity.
Benefits of CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry
Comprehensive Understanding: The notes provide a detailed explanation of key concepts like electrolysis, conductivity, and molar conductivity, helping students grasp the fundamental principles of electrochemistry.
Clear Definitions and Examples: Definitions of important terms (e.g., electrolytes, strong vs. weak electrolytes) and examples are included to clarify these concepts, making it easier to understand and remember them.
Simplified Conceptualization: Complex topics are broken down into simpler parts which helps in understanding the underlying mechanisms of electrochemical processes, such as the movement of ions and their impact on conductivity.
Quick Revision: The concise format of the notes allows for quick revisions, making it easier to review important topics and formulas before exams.
CBSE Class 12 Chemistry Notes Chapter 3 Electrochemistry FAQs
What is electrochemistry?
What is an electrolyte?
What is the difference between strong and weak electrolytes?
What is electrolysis?
What are the electrodes in electrolysis?










