
CBSE Class 12 Chemistry Notes Chapter 5: Surface Chemistry is a important topic in the CBSE Class 12 Chemistry syllabus. Understanding surface chemistry can significantly enhance your grasp of various chemical processes and applications.
Our detailed CBSE Class 12 Chemistry Notes for Chapter 5 Surface Chemistry are designed to help you master this subject effectively. They cover detailed explanations of surface phenomena, including adsorption types (physisorption and chemisorption), the role of catalysts, and the properties of colloidal systems. By using these chapter-wise notes, you can simplify complex concepts and improve your performance in the CBSE Class 12 Chemistry exam.CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry Overview
CBSE Class 12 Chemistry Notes for Chapter 5 Surface Chemistry are prepared by subject experts of Physics Wallah to provide a detailed overview of this important topic. These notes provide a detailed examination of surface chemistry concepts, including adsorption, catalysis, and the properties of colloidal systems. By breaking down complex ideas into clear and understandable segments the notes ensure that students grasp the nuances of surface phenomena effectively. Whether you're revising for exams or seeking to deepen your understanding, these expert-prepared notes are an invaluable resource for mastering surface chemistry and achieving academic success in your CBSE Class 12 Chemistry course.CBSE Class 12 Chemistry Notes Chapter 5 PDF
The PDF for CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry is available below. This resource provides a thorough overview of the chapter, covering essential topics such as adsorption, catalysis, and colloids. Access the PDF using the link provided to enhance your study and ensure a solid foundation in surface chemistry.CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry PDF
CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry
Here we have provided CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry-Surface Chemistry
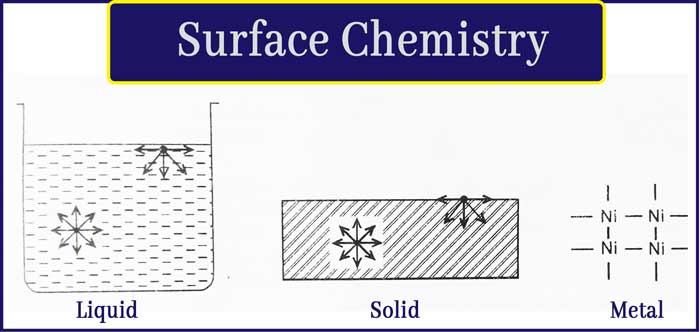 As described in the notes, Surface Chemistry is the branch of chemistry that focuses on the properties and behavior of surfaces and interfaces. It explain phenomena such as adsorption on solid or liquid surfaces and colloidal properties, which are critical aspects of surface effects.
Surface Chemistry examines chemical reactions that occur at surfaces or interfaces, playing a crucial role in various practical applications. For example, it is fundamental in processes like creating high vacuums, manufacturing gas masks, and understanding catalytic reactions.
The principles of Surface Chemistry are widely utilized across industries and scientific research to optimize and innovate in fields ranging from environmental technology to industrial manufacturing.
As described in the notes, Surface Chemistry is the branch of chemistry that focuses on the properties and behavior of surfaces and interfaces. It explain phenomena such as adsorption on solid or liquid surfaces and colloidal properties, which are critical aspects of surface effects.
Surface Chemistry examines chemical reactions that occur at surfaces or interfaces, playing a crucial role in various practical applications. For example, it is fundamental in processes like creating high vacuums, manufacturing gas masks, and understanding catalytic reactions.
The principles of Surface Chemistry are widely utilized across industries and scientific research to optimize and innovate in fields ranging from environmental technology to industrial manufacturing.
Adsorption
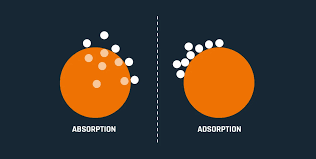
Adsorption is a process where molecular species (gases or liquids) become concentrated on the surface of a solid or liquid, rather than being distributed throughout the bulk. This occurs when the concentration of these species is higher at the surface than in the surrounding medium.
In this context:- Adsorbent refers to the solid or liquid that provides the surface for adsorption. It is the material on which the adsorption takes place.
- Adsorbate refers to the substance that is adsorbed onto the surface of the adsorbent. This can be a gas or a liquid that adheres to the surface.
- The surface area of the solid: A larger surface area increases the extent of adsorption.
- The temperature of the gas: Temperature changes can affect the adsorption capacity.
- The nature of the gas and the pressure: Different gases interact differently with surfaces, and pressure variations can influence the adsorption process.
- The nature of the solid: The chemical and physical properties of the adsorbent play a significant role in adsorption.
Definitions:
- Adsorption: The presence of a substance at an interface in a different concentration compared to the adjoining bulk is referred to as adsorption.
- Adsorbate: The substance that adheres to the surface of another material is known as the adsorbate.
- Adsorbent: The material or substance present in bulk on which adsorption occurs is called the adsorbent.
- Desorption: The process of removing an adsorbed substance from the surface to which it was adhered is known as desorption.
Mechanism of Adsorption
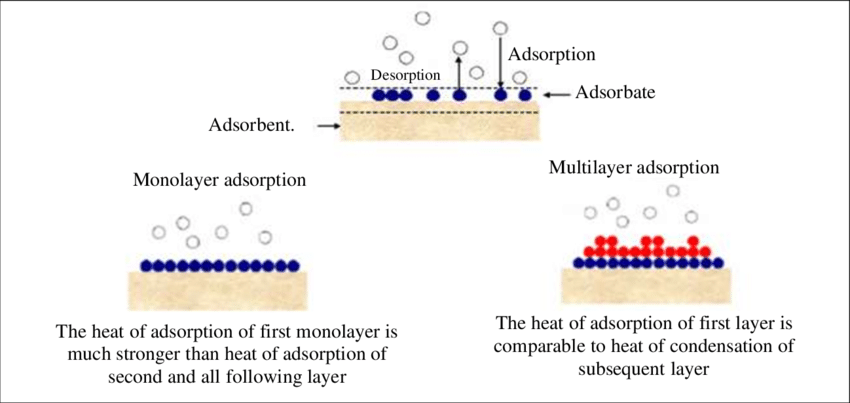 Adsorption is an
exothermic process
, meaning it releases energy. The heat released when one mole of adsorbate is adsorbed onto the adsorbent is referred to as the enthalpy of adsorption, and this value is typically negative. This negativity in enthalpy arises because the adsorption process restricts the movement of adsorbate molecules, leading to a decrease in entropy. At constant temperature and pressure, adsorption tends to occur spontaneously.
Adsorption is an
exothermic process
, meaning it releases energy. The heat released when one mole of adsorbate is adsorbed onto the adsorbent is referred to as the enthalpy of adsorption, and this value is typically negative. This negativity in enthalpy arises because the adsorption process restricts the movement of adsorbate molecules, leading to a decrease in entropy. At constant temperature and pressure, adsorption tends to occur spontaneously.
Types of Adsorption
Physical Adsorption (Physisorption)
Physical adsorption, or physisorption, involves the adsorption of adsorbate molecules onto the adsorbent surface through weak van der Waals forces. This type of adsorption is non-specific and reversible.Characteristics of Physisorption:
- Non-Specific Nature: Any gas can be adsorbed onto the surface, and the extent of adsorption depends on the nature of the gas and the surface area of the adsorbent.
- Reversibility: Physisorption is reversible. Increasing the pressure of the gas enhances adsorption, while decreasing the pressure allows desorption. Similarly, increasing temperature tends to reduce adsorption as per Le Chatelier’s principle.
- Dependence on Surface Area: Adsorption is more effective on porous and finely divided materials due to their larger surface area.
- No Energy Activation Required: Physical adsorption does not require additional energy for activation and occurs more readily at lower temperatures.
Chemical Adsorption (Chemisorption)
Chemical Adsorption or Chemisorption occurs when the forces holding the adsorbate molecules to the adsorbent are as strong as chemical bonds. This type of adsorption involves a more specific interaction between the adsorbate and the adsorbent compared to physical adsorption.
Characteristics of Chemisorption:
- Definite and Irreversible: Chemisorption is typically a one-time process where adsorbate molecules form strong bonds with the adsorbent, making it irreversible.
- Surface Area Dependence: Like physical adsorption, chemisorption also increases with surface area, but due to its chemical nature, it requires specific surface sites.
- Strong Attractive Forces: The forces involved are akin to chemical bonds, resulting in a strong interaction between the adsorbent and adsorbate.
- Formation of Unimolecular Layers: It often results in the formation of a single molecular layer on the surface.
- Exothermic Process: Chemisorption releases energy, and while it is exothermic, the process rate increases with temperature and pressure.
- Slow at Low Temperatures: Chemisorption generally occurs slowly at low temperatures but accelerates with higher temperatures and pressures.
- Specific Activation Energy: This process requires specific activation energy due to the formation of chemical bonds.
Factors Affecting Adsorption of Gases by Solids
- Nature and Surface Area of Adsorbent: Different solids adsorb the same gas to varying extents. An increased surface area enhances the volume of gas adsorbed.
- Nature of the Gas: Gases are adsorbed differently based on their critical temperatures. Gases with higher critical temperatures are more easily liquefied and thus more readily adsorbed.
- Temperature: Adsorption generally decreases as the temperature increases, as higher temperatures provide energy that competes with the adsorption process.
- Pressure: At constant temperature, the adsorption of a gas increases with pressure, as higher pressure leads to a higher concentration of gas molecules at the surface.
- Potassium chlorate, when heated, powerfully decomposes slowly, giving dioxygen. The decomposition between 653 – 873 K.
- Activity:
- Selectivity:
Homogeneous Catalysis: Homogeneous catalysis involves a catalyst that is in the same phase as the reactants. For example, in the hydrolysis of glucose, an acid acts as a catalyst and is present in the same aqueous phase as the glucose. This type of catalysis ensures that the catalyst and reactants are uniformly mixed, leading to efficient interaction and reaction.
Heterogeneous Catalysis: In heterogeneous catalysis, the catalyst is in a different phase from the reactants. A classic example is the synthesis of ammonia from hydrogen and nitrogen using iron as a catalyst. Here, the reactants (gases) and the catalyst (solid iron) are in different phases, which requires the reactants to diffuse to the surface of the solid catalyst for the reaction to occur.
Positive Catalysis: Positive catalysis refers to the acceleration of a chemical reaction by a catalyst. For instance, colloidal platinum acts as a positive catalyst in the decomposition of hydrogen peroxide, facilitating the reaction:
2H2O2(l) → 2H2O2(l) + O2(g)Photocatalysis: Photocatalysis involves a catalyst that is activated by light. The catalyst absorbs light energy and enters an excited state, which facilitates the chemical reaction. This process is commonly used in applications such as environmental cleanup and solar energy conversion.
Induced Catalysis: Induced catalysis refers to the phenomenon where the presence of a catalyst influences the rate of other reactions that would not occur under ordinary conditions. This concept highlights how certain conditions or catalysts can enable reactions that otherwise would not proceed.
Classification of Colloids
Colloids are substances with particle sizes in the range of 1-1000 nm, which exhibit properties intermediate between those of true solutions and suspensions. They can be classified based on the physical state of the dispersed phase and dispersed medium, as well as other characteristics.Based on Physical State of the Dispersed Phase and Dispersed Medium
| S.No | Dispersed Phase | Dispersed Medium | Name of Colloid | Examples |
|---|---|---|---|---|
| 1 | Solid | Solid | Solid sol | Gemstones, some coloured glasses |
| 2 | Solid | Liquid | Sol | Muddy water, paints, cell fluids |
| 3 | Solid | Gas | Aerosol | Smoke, dust |
| 4 | Liquid | Solid | Gel | Cheese, jelly |
| 5 | Liquid | Liquid | Emulsion | Milk, hair cream, butter |
| 6 | Liquid | Gas | Aerosol | Fog, mist, cloud, insecticide sprays |
| 7 | Gas | Solid | Solid sol | Pumice stone, foam rubber |
| 8 | Gas | Liquid | Foam | Froth, soap lather, whipped cream |
Based on Characteristics of Interaction Between Dispersed Phase and Dispersed Medium
Lyophilic Colloids (Liquid-Loving)
- Description: Formed when substances that are affinity for the dispersion medium directly form colloids. They are stable and reversible. If the dispersion phase is separated, it can be recombined with the dispersion medium to reform the sol.
- Examples: Gum, gelatine, starch, rubber.
Lyophobic Colloids (Liquid-Hating)
- Description: These colloids are difficult to form and are irreversible. They have little to no affinity for the dispersion medium and are often formed using specific methods.
- Examples: Metals like silver (Ag) and gold (Au), hydroxides like aluminum hydroxide (Al(OH)₃) and ferric hydroxide (Fe(OH)₃), metal sulfides like arsenic sulfide (As₂S₃).
Based on Types of Particles of the Dispersed Phase
Multimolecular Colloids
- Description: Formed when many atoms or molecules combine to form particles in the colloidal size range (1-1000 nm).
- Examples: Sulphur sol, gold sol.
Macromolecular Colloids
- Description: Consist of large molecules or macromolecules that are in the colloidal size range when dissolved in a suitable solvent. These solutions resemble true solutions but are colloidal in nature.
- Examples: Natural macromolecules like starch, cellulose, and proteins; synthetic macromolecules like nylon, synthetic rubber, polythene, and polystyrene.
Associated Colloids (Micelles)
- Description: These substances behave as normal electrolytes at low concentrations but form colloidal aggregates called micelles at high concentrations.
- Examples: Surfactants and detergents, which form micelles in solutions.
Benefits of CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry
- Comprehensive Understanding : The notes provide a detailed overview of surface chemistry, including key concepts such as adsorption, catalysis, colloids, and surface phenomena. This helps students grasp complex topics more effectively.
- Clear Definitions and Explanations : Important terms and concepts like adsorption isotherms (Freundlich and Langmuir), types of adsorption, and colloid classifications are explained in a clear and concise manner making it easier for students to understand and remember.
- Visual Aids : Diagrams, tables, and graphs included in the notes help illustrate concepts such as the Freundlich adsorption isotherm, types of colloids, and mechanisms of catalysis. Visual aids can enhance comprehension and retention of material.
- Conceptual Clarity : With detailed explanations of complex processes and phenomena such as catalytic cycles and the formation of micelles, the notes help in clarifying difficult concepts, leading to better conceptual understanding.
CBSE Class 12 Chemistry Notes Chapter 5 Surface Chemistry FAQs
What is surface chemistry?
What are the two main types of adsorption?
What is the difference between lyophilic and lyophobic colloids?
What are micelles?
What is the role of surface chemistry in catalysis?










