
CBSE Class 12 Chemistry Notes Chapter 2: CBSE Class 12 Chemistry Notes for Chapter 2 Solutions are important for students studying Chemistry, as mastering the concepts in this chapter is important for understanding various applications in chemistry.
These notes cover key topics such as the types of solutions, concentration expressions, colligative properties, and the methods for preparing and analyzing solutions. These notes are designed to simplify complex topics and provide clear explanations helping students grasp challenging concepts with ease. By using these notes students can enhance their understanding and improve their performance in Chemistry exams ensuring they achieve better results and a deeper comprehension of the subject.CBSE Class 12 Chemistry Notes Chapter 2 Solutions Overview
CBSE Class 12 Chemistry Notes for Chapter 2 Solutions are prepared by subject experts of Physics Wallah. These notes cover key topics like types of solutions, how to measure concentration and colligative properties.They explain these concepts in a clear and easy-to-understand way. These notes help students grasp difficult topics better and prepare effectively for their Chemistry exams.
CBSE Class 12 Chemistry Notes Chapter 2 PDF
CBSE Class 12 Chemistry Notes for Chapter 2, Solutions are available in a comprehensive PDF format. This PDF covers all important topics related to solutions, including types, concentration measurements, and colligative properties, with clear explanations and examples. You can access the PDF through the link below to enhance your understanding and preparation for the Chemistry exam.CBSE Class 12 Chemistry Notes Chapter 2 Solutions PDF
CBSE Class 12 Chemistry Notes Chapter 2 Solutions
Here we have provided CBSE Class 12 Chemistry Notes Chapter 2 Solutions-Solutions
A solution is a homogeneous mixture formed when two or more substances are combined, potentially in different physical states. The substances that make up a solution are known as its components. In a binary solution, which consists of two components, the solvent is the substance present in the larger amount, while the solute is the component present in a smaller amount.Classification of Solutions:
Based on Physical State:
- Aqueous Solution: If water is the solvent, the solution is called an aqueous solution.
- Non-Aqueous Solution: If the solvent is not water, the solution is termed non-aqueous.
Based on Amount of Solute:
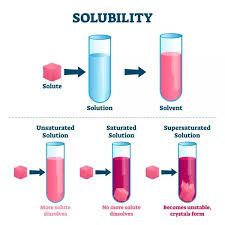
- Unsaturated Solution: This type of solution can dissolve more solute at a given temperature.
- Saturated Solution: In this solution, no more solute can be dissolved at a specific temperature.
- Supersaturated Solution: This contains more solute than what would normally be dissolved at that temperature, often achieved by altering the temperature or pressure.
Solubility
Solubility is defined as the maximum amount of solute that can be dissolved in a specific amount of solvent (usually 100 grams) at a given temperature. It indicates how well a solute dissolves in a solvent to form a solution. The solubility of a solute in a liquid is influenced by several factors:
Nature of the Solute: Different solutes have varying degrees of solubility based on their chemical properties and interactions with the solvent.
Nature of the Solvent: The solvent's properties, such as its polarity and ability to interact with the solute, significantly impact solubility.
Temperature: Solubility generally increases with temperature for most solid solutes, but this can vary for different substances.
Pressure: For gases, solubility in a liquid is directly proportional to the pressure of the gas above the liquid, as described by Henry's Law.
Henry’s Law
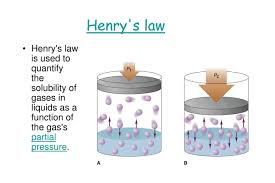
Henry’s Law states that the partial pressure (P) of a gas in the vapor phase is directly proportional to the mole fraction (x) of that gas in the solution.
Applications:
Soft Drinks and Soda Water: Carbon dioxide (CO₂) is added under high pressure to increase its solubility, enhancing the fizz in beverages.
Deep-Sea Diving: To reduce the risk of decompression sickness (the bends), divers breathe a mixture of oxygen and helium. Helium, being less soluble, minimizes the risk of nitrogen bubbles forming in the bloodstream.
High Altitudes: At higher altitudes, the partial pressure of oxygen is lower, resulting in decreased oxygen levels in the blood and leading to conditions like hypoxia or "anoxia," which can affect climbers and travelers at high elevations.
Concentration of Solutions
The concentration of a solution refers to the amount of solute present in a given quantity of solution. It is a measure of how much solute is dissolved in a solvent. Solutions can be categorized based on their concentration into two main types:Dilute Solution: A dilute solution contains a relatively small amount of solute compared to the solvent. In such solutions, the concentration of the solute is low, meaning that there is a high proportion of solvent relative to solute.
Concentrated Solution: A concentrated solution contains a large amount of solute relative to the solvent. In these solutions, the solute concentration is high, meaning that there is a smaller proportion of solvent compared to solute.
Methods of Expressing Concentration of Solutions
There are several ways to express the concentration of solutions, each suitable for different contexts and precision requirements:Percentage by Weight (w/w%) : This is the ratio of the weight of the solute to the total weight of the solution, multiplied by 100. It indicates how much of the solute is present in 100 grams of solution.
Percentage by Volume (w/V%) : This can refer either to the weight of the solute in 100 mL of solution or the volume of the solute in 100 mL of solution.
Mole Fraction (x) : This is the ratio of the number of moles of a component to the total number of moles of all components in the solution.
Parts Per Million (ppm) : This measures the amount of solute in one million parts of the solution, useful for trace quantities.
Molarity (M) : This is the number of moles of solute per liter of solution.
Molality (m) : This measures the number of moles of solute per kilogram of solvent.
Normality (N) : This is the number of gram-equivalents of solute per liter of solution.
Formality (F) : This is the number of formula weights of solute per liter of solution.
Mass Fraction : This represents the mass of a component divided by the total mass of the solution, indicating the proportion of the solute in the solution.
Demal (D) : Represents one mole of solute per liter of solution at 0°C.
Raoult’s Law
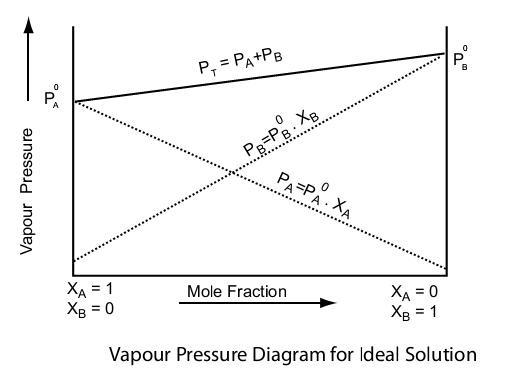 Raoult’s Law describes how the vapor pressure of a solvent is affected when a non-volatile solute is added. The law states that the relative lowering of the vapor pressure of the solvent is directly proportional to the mole fraction of the solute in the solution.
Raoult’s Law describes how the vapor pressure of a solvent is affected when a non-volatile solute is added. The law states that the relative lowering of the vapor pressure of the solvent is directly proportional to the mole fraction of the solute in the solution.
P total = p 1 0 x 1 +p 2 0 x 2
Ideal Solutions
An ideal solution is one that perfectly follows Raoult’s Law across all concentrations. In an ideal solution, the intermolecular forces between the molecules of the solute and solvent are similar to those between the molecules of the pure substances. This means that the interactions between the solute and solvent molecules do not differ significantly from the interactions within each component in its pure state.Types of Deviations from Raoult’s Law
Positive Deviations:
- Characteristics: In a solution with positive deviations, the vapor pressure of the solution is higher than what Raoult’s Law predicts. This occurs because the intermolecular forces between the solute and solvent molecules are weaker than those between the molecules of the pure substances.
- Reason: The weaker solute-solvent interactions lead to higher vapor pressures because the solute molecules disrupt the intermolecular forces between the solvent molecules, making it easier for molecules to escape into the vapor phase.
- Example: A common example is a mixture of acetone and chloroform. The interaction between acetone and chloroform is weaker than the interactions in pure acetone or pure chloroform.
Negative Deviations:
- Characteristics: In a solution with negative deviations, the vapor pressure of the solution is lower than what Raoult’s Law predicts. This happens because the intermolecular forces between the solute and solvent molecules are stronger than those in the pure substances.
- Reason: Stronger solute-solvent interactions result in a lower vapor pressure. The strong interactions between the solute and solvent molecules reduce the number of molecules escaping into the vapor phase.
- Example: A typical example is a solution of water and hydrochloric acid. The strong hydrogen bonding between water molecules and the ionic interactions with HCl lead to lower vapor pressure than expected.
Konowaloff's Rule
Konowaloff's Rule states that at a fixed temperature, the vapor phase in equilibrium with a solution will always be richer in the more volatile component compared to the solution phase. In simpler terms, the mole fraction of the more volatile component is always greater in the vapor phase than it is in the liquid solution phase.CBSE Class 12 Chemistry Notes Chapter 2 Solutions FAQs
What is a solution?
What are the types of solutions based on physical state?
How is solubility defined?
What factors affect solubility?
What is Raoult’s Law?










