
CBSE Class 12 Chemistry Notes Chapter 13: CBSE Class 12 Chemistry Notes Chapter 13 tells us about Amines, that are organic compounds that are created by substituting alkyl or aryl groups for one or more hydrogen atoms in ammonia (NH3). Based on the quantity of alkyl/aryl groups bonded to the nitrogen atom, they are divided into three categories: primary (1°), secondary (2°), and tertiary (3°) amines.
Amines have hydrogen bonds, which affect how soluble and boiling points they are. Basicity is influenced by their structure; aliphatic amines are more basic than aromatic ones. Amines are essential for the production of polymers, drugs, chemical synthesis, and colours. They can be made by reducing nitro compounds, amides, or by breaking down Hoffmann bromamide and synthesising Gabriel phthalimide.CBSE Class 12 Chemistry Notes Chapter 13 Overview
Amines are organic compounds that are produced from ammonia (NH3) in which alkyl or aryl groups have taken the place of one or more hydrogen atoms. A classifier for amines is the number of substituents on nitrogen; 1°, 2°, or 3° indicates a primary amine. Depending on the type of carbon chain that is joined to the nitrogen atom, they can be either aromatic or aliphatic. Because nitrogen has a single pair of electrons that can take protons, amines have basic characteristics. The type of the solvent and substituents affects how basic they are. Amines can be produced by reducing amides, nitro compounds, and nitriles, among other things. They also go through the Hoffmann degradation, acylation, and alkylation processes. Aniline is one of the aromatic amines that exhibits electrophilic substitution reactions. Amines are used extensively in the manufacturing of polymers, medications, and colours. They are also essential for metabolic processes like neurotransmission.CBSE Class 12 Chemistry Notes Chapter 13 PDF
Here we have provided CBSE Class 12 Chemistry Notes Chapter 13 Amines pdf for the ease of the students so that they can download it and access it offline.CBSE Class 12 Chemistry Notes Chapter 13 PDF
CBSE Class 12 Chemistry Notes Chapter 13 Amines
Here we have provided CBSE Class 12 Chemistry Notes Chapter 13 Amines - Ammonia is the starting point for amines, which are created by substituting alkyl or aryl groups for hydrogen atoms. A primary amine is created by replacing one hydrogen atom. While RNR′R′′, R2NR′, or R3N characterize tertiary amines, R2NH or R-NHR′ indicates the structure of secondary amines. Tritary and secondary amines are referred to as mixed amines if their aryl or alkyl groups are connected to distinct groups, while they are referred to as simple amines if they are coupled to the same group. Amines function as Lewis bases because they have one unshared electron pair on the nitrogen atom. They can be made of imides, nitro compounds, amides, or halides.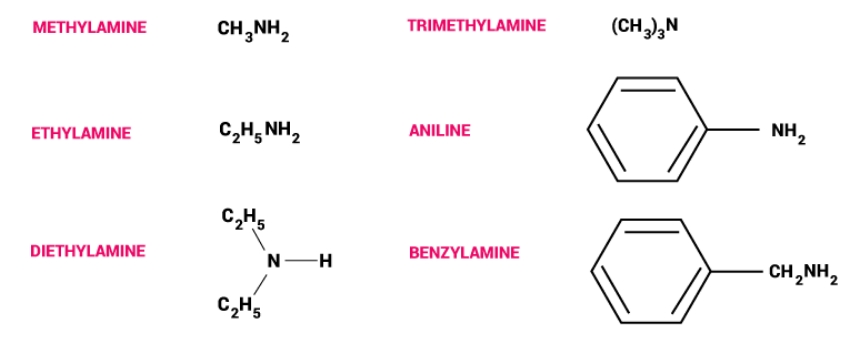
Alkyl Amines
A mixture of steric, H-bonding, and electron-releasing variables impact the stability of substituted ammonium cations in protic polar liquids, which in turn affects the basic character of amines. Alkyl amines have a stronger base than ammonia.Aromatic Amines
The fundamental properties of aromatic amines rely on electron release and withdrawing groups. Phenoluenesulphonyl chloride is used to separate and identify amines, including primary, secondary, and tertiary ones. Aromatic amine reactivity is regulated by the acylation process.Nomenclature of Amines by IUPAC System:
Common Names of Amines:
Alkylamine is the name of an aliphatic amine in the standard system, which is formed by prefixing the alkyl group to amine. Primary amines are under consideration. The prefix di or tri is appended to the name of the alkyl group in secondary and tertiary amines whenever two or more groups are the same. Alphabetical order is used for writing alkyl groups in mixed amine cases. CH3CH2-CH2-NH2 Propylamine CH3CH(-NH2)-CH3 Isopropylamine IUPAC System: According to the IUPAC system, amines are referred to as alkanamines when the word "amine" is substituted for the letter "e" in alkane. Two such examples are methanamine (CH3NH2) and ethane amine (C2H5NH2). For the purpose of designating higher member hydrocarbons, the longest chain with an amino group is chosen. A lower number is assigned to the amino group that is joined to the C atom. If more than one amino group is present in the parent chain at different positions, their locations are identified by assigning distinct numbers to the carbon atoms carrying the -NH2 and by attaching an appropriate prefix, such as di, tri, etc., to the amine. Nonetheless, the hydrocarbon part's suffix letter "e" is kept. For example: (Ethane 1,2 diamine) H2N-CH2-CH2-NH2 Every alkyl group bonded to the N atom is classified as an N-alkyl group.Aryl Amines
In aryl amines, the NH2 group is joined to the benzene ring directly. The most basic aryl amine is C6H5NH2. Another name for it is aniline. The IUPAC method states that when designating aryl amines, the suffix "amine" takes the place of "e," which stands for arene. For example, C6H5-NH2 is named benzenamine. Preparation of Amines: The methods for making amines are as follows:- Decrease in nitro substances.
- Ammonolysis of halides with alkali.
- Decrease in nitriles.
- Decrease in amides.
- Gabriel synthesis of phthalimide.
- Hoffmann bromamide breakdown process.
Reduction of nitro compounds
Nitrogen compounds are reduced to amines by reducing with metals in an acidic media or by passing hydrogen gas through finely divided nickel, palladium, or platinum. R- NO2 + 3H2 → R-NH2 + 2H2O, In the presence of Ni, Pt or Pd Ar- NO2 + 3H2 → Ar- NH2 + 2H2O Alkanamines can also be obtained by reducing nitro alkanes. Since the hydrolysis of FeCl2 during the reaction produces hydrochloric acid, reduction using iron scrap and hydrochloric acid is preferred. Thus, the reaction can be started with a tiny amount of hydrochloric acid. Nitro (-NO2) compounds can also be reduced using concentrated HCL and active metals such as Fe, Sn, Zn, etc. R- NO2 + 3H2 → R- NH2 + 2H2O, In the presence of Sn/HCl Ar- NO2 + 3H2 → Ar- NH2 + 2H2OAmmonolysis of alkyl halides
Using the SN2 process, alkyl halides are nucleophilically substituted, resulting in the formation of primary amines from NH3. In alkyl or benzyl halides, the carbon-halogen link can be effectively broken by a nucleophile. As a result, in an alkyl or benzyl halide reaction with an ethanolic ammonia solution, the halogen atom is invariably replaced by an amino (-NH2) group in a nucleophilic substitution process. Ammonolysis is the term for this C–X bond breakage that occurs when ammonia is used as a catalyst. At 373 K, the reaction is carried out in a sealed tube. Thus created, the main amine exhibits nucleophilic behaviour. It can then react with an alkyl halide to produce quaternary ammonium salt, secondary and tertiary amines, and so on. When the ammonium salt reacts with a strong base, the free amine is produced. Disadvantages : A primary amine is formed as the main product when ammonia is taken in excess. When alkyl halide is used in excess, the quaternary ammonium salt is formed as the main product. Reduction of nitriles When nitriles are reduced using lithium aluminium hydroxide (LiAlH4) or catalytically hydrogenated, primary amines are produced. This reaction is used to prepare amines with one carbon atom more than the beginning amine, or an ascent of the anime series. Reduction of amides: On reduction with lithium aluminium hydride, the amides give amines. Gabriel phthalimide synthesis: First, primary amines are prepared by the Gabriel synthesis. The potassium salt of phthalimide is created when ethanolic potassium hydroxide and phthalimide are combined. The related primary amine is produced via heating with alkyl halide and alkaline hydrolysis. Because aryl halides do not undergo nucleophilic substitution with the anion produced by phthalimide, this technique is unable to synthesise primary aromatic amines. Hoffmann bromamide degradation reaction: Students study the process Hoffmann developed to prepare primary amines by combining an amide and bromine in an aqueous solution of sodium hydroxide. An alkyl or aryl group migrates from the amide's carbonyl carbon to the nitrogen atom during this reaction. One carbon is missing from the amine that is thus produced compared to the amide. Physical Properties of Amines :Physical state and smell:
The lower aliphatic amines smell like fish and are gaseous. At normal temperature, primary amines with three carbon atoms or more are liquids, and those with more are solids. The lower members of the arylamine family are liquid, whereas the higher members are solids. Normally colourless, aniline and other arylamines become coloured when stored outside due to air oxidation.Solubility:
Water molecules can establish hydrogen bonds with lower aliphatic amines. They dissolve in water because of this. A decrease in an amine's solubility in water is caused by an increase in the molar mass of the hydrophobic alkyl component. Water does not dissolve higher amines by nature. A few organic solvents, including ether, benzene, and alcohol, can dissolve amines easily. Alcohols and amines generate stronger intermolecular hydrogen interactions due to alcohols' higher polarity.Boiling Point:
Because of polar hydrogen bonds that form between one molecule's nitrogen and the other's hydrogen, primary and secondary amines frequently interact with one another intermolecularly. Because there are two hydrogen atoms present in primary amines, the intermolecular interaction is more pronounced than in secondary amines. Due to the lack of a free hydrogen atom for bonding, tertiary amines do not exhibit intermolecular interaction. The following is the order in which amines boil: First, Second, Third, Tertiary.Methods of Preparation of Diazonium Salts:
Benzenediazonium chloride is produced at 273-278 K when aniline and nitrous acid combine. Hydrochloric acid and sodium nitrite react to produce nitrous acid in the reaction mixture. The process of turning primary aromatic amines into diazonium salts is known as diazotisation.Physical Properties:
The crystalline solid benzoenediazonium chloride is colourless. In the cold, it is easily soluble and stable, but when heated, it reacts with water. It breaks down rapidly when it's dry. Certain diazonium salts, including benzenediazonium fluoroborate, are stable at room temperature and insoluble in water.Benefits of CBSE Class 12 Chemistry Notes Chapter 13
CBSE Class 12 Chemistry notes on Chapter 13, "Amines," provide several benefits for students:Concept Clarity : The notes simplify complex topics, ensuring a clear understanding of amine structures, properties, and reactions.
Time-Saving : Well-organized notes allow students to revise the chapter quickly, especially before exams.
Comprehensive Coverage : Important concepts like classification, preparation methods, chemical reactions, and applications of amines are covered concisely.
Easy Recall : Key points, definitions, and equations are presented in a way that aids in easier memorization and recall during exams.
Exam Preparation : The notes include commonly asked questions, which helps students prepare effectively for board exams by focusing on important topics.
Application-Based Learning : Understanding amines is crucial for real-life applications, such as in pharmaceuticals and industrial processes, making the notes relevant for future studies and careers.
CBSE Class 12 Chemistry Notes Chapter 13 FAQs
What are amines used for?
What is another name for amines?
Are amines basic or acidic?
Is amine organic or inorganic?










