
CBSE Class 12 Chemistry Notes Chapter 12: The important organic molecules aldehydes, ketones, and carboxylic acids are covered in Chapter 12 of CBSE Class 12 Chemistry. Ketones have the carbonyl group (C=O) joined to two alkyl groups, whereas aldehydes have the carbonyl group (C=O) bonded to a hydrogen atom. A carboxyl group (-COOH) is present in carboxylic acids.
Their structure, nomenclature, and physical characteristics, such as solubility and boiling points, are examined in this chapter. It also covers key reactions like nucleophilic addition and oxidation and preparation techniques like the oxidation of alcohols. Additionally highlighted are the uses of these chemicals in the pharmaceutical, solvent, and perfume industries.CBSE Class 12 Chemistry Notes Chapter 12 Overview
Aldehydes, ketones, and carboxylic acids are the three main groups of organic compounds with the carbonyl group (C=O) that are covered in Chapter 12 of CBSE Class 12 Chemistry. Ketones have the carbonyl group connected to two alkyl or aryl groups, while aldehydes have it attached to a hydrogen atom and one of these groups. On the other hand, carboxylic acids have a carboxyl group (-COOH) made composed of a hydroxyl and a carbonyl group. The chapter explores these compounds' structure and nomenclature, outlining IUPAC and accepted naming practices. It looks at the physical characteristics that come from being polar, like boiling temperatures, solubility, and dipole moments. Oxidation of alcohols and ozonolysis of alkenes are important procedures for preparing aldehydes and ketones, whereas carboxylic acids are frequently made by oxidising aldehydes and primary alcohols. Among the reactions discussed are decarboxylation, esterification, and reactions with bases, as well as nucleophilic addition in aldehydes and ketones. A thorough discussion of these compounds' chemical reactivity is included, highlighting their industrial importance in the manufacturing of solvents, fragrances, dyes, and medications.CBSE Class 12 Chemistry Notes Chapter 12 PDF
Here we have provided CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids pdf for the ease of students so that they can download it and access it offline.CBSE Class 12 Chemistry Notes Chapter 12 PDF
CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Here we have provided CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids - The carbonyl group ()C=O) is bound to two carbon atoms in ketones and to carbon and hydrogen in aldehydes.Haloform reaction - When sodium hypohalite is added to aldehydes and ketones with at least one methyl group [3-α hydrogen] attached to the carbonyl carbon atom, it oxidises the compounds to sodium salts of the corresponding carboxylic acids, which have one carbon atom less than the carbonyl compound. Haloform is produced by converting the methyl group.
Nature of Carbonyl Group
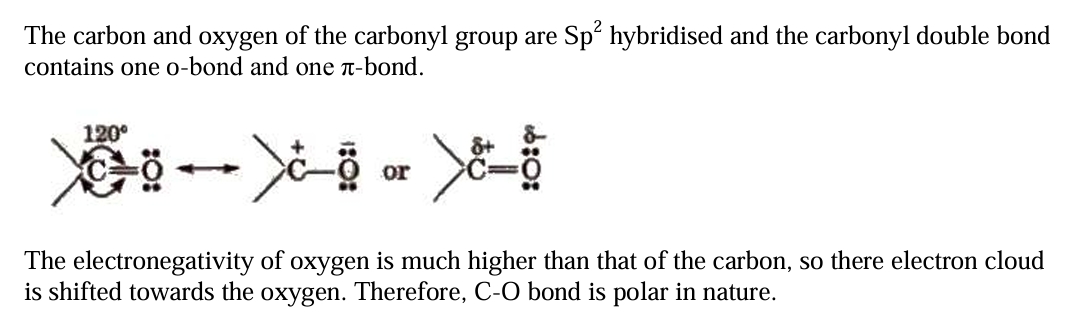
Nomenclature
Nomenclature in organic chemistry refers to the systematic naming of compounds based on their structure, following the rules established by the International Union of Pure and Applied Chemistry (IUPAC).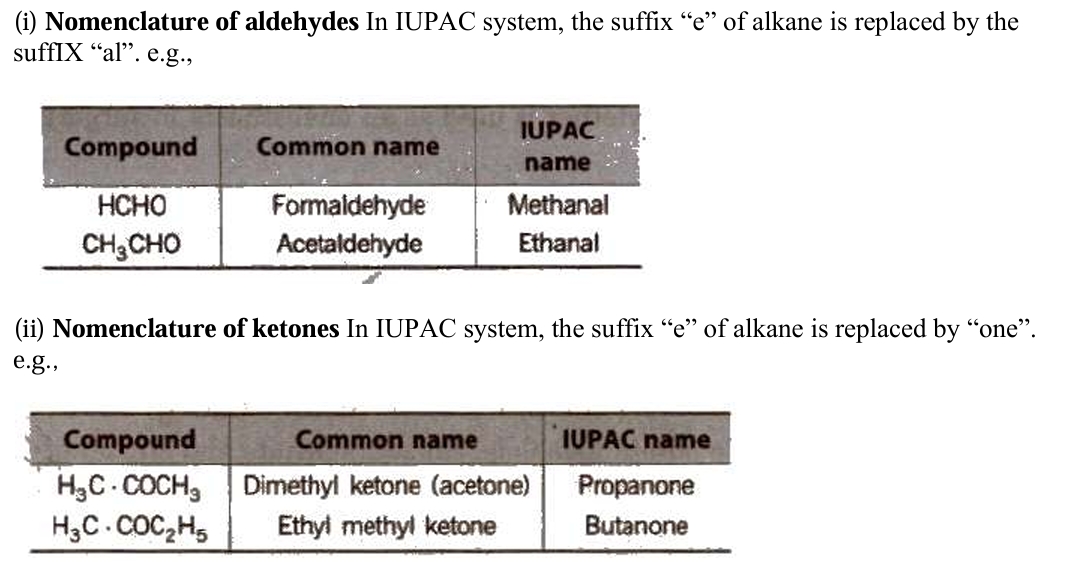
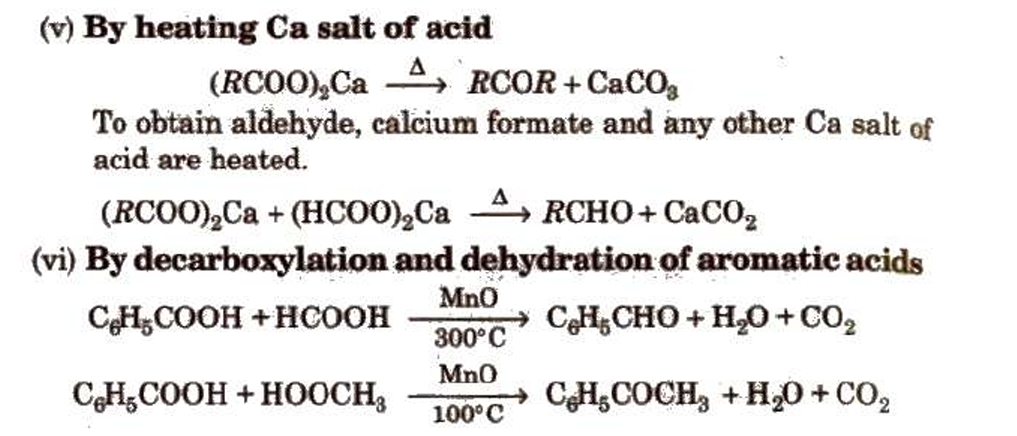
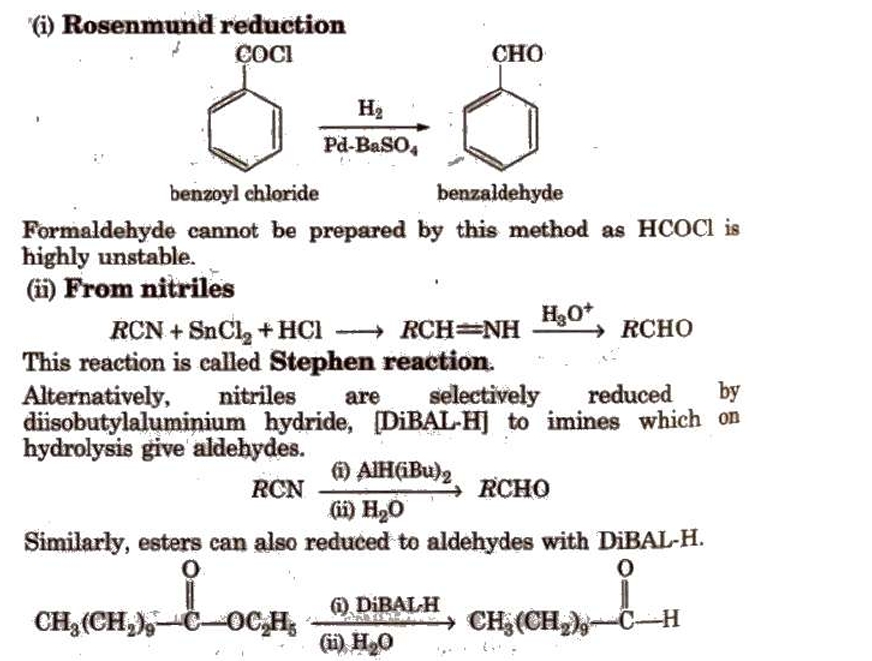
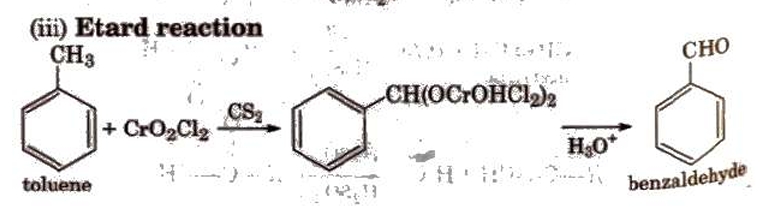
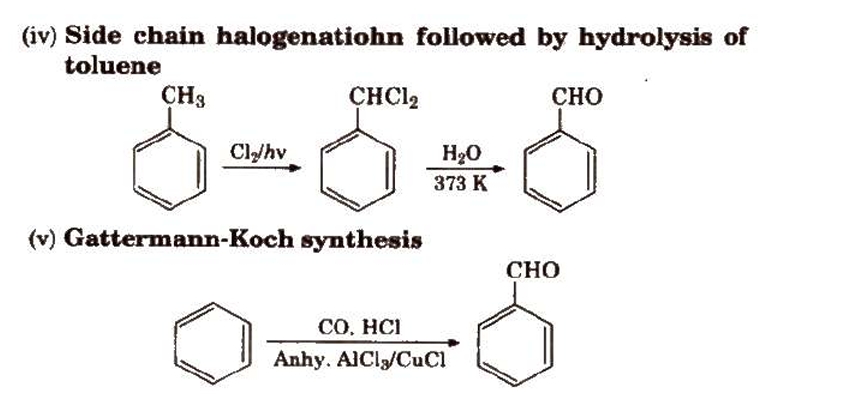
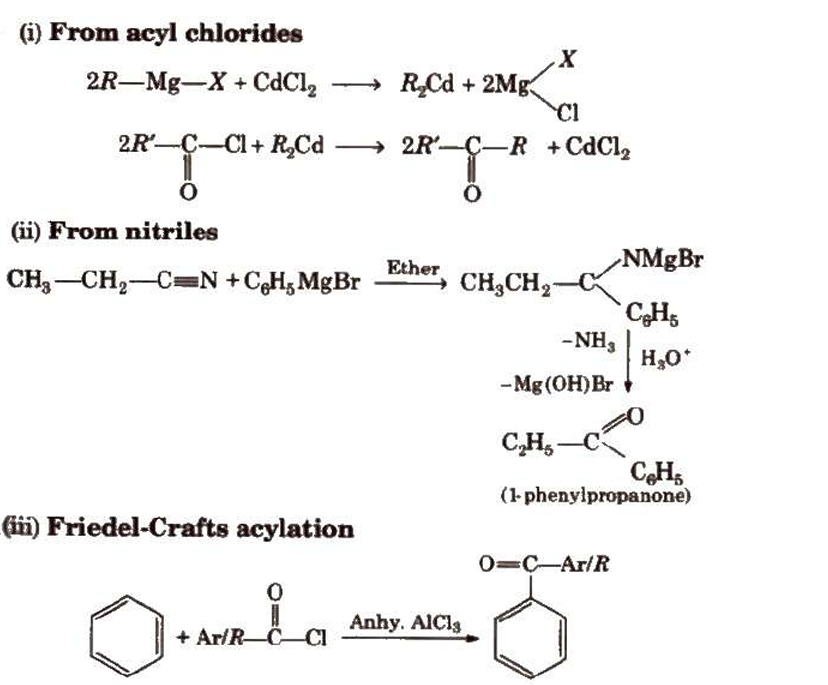
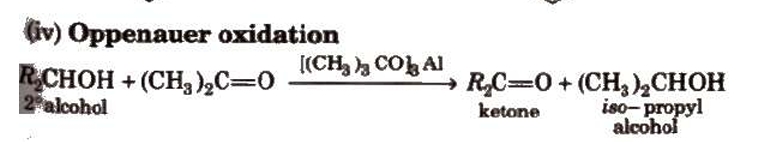
Preparation of Aldehydes and Ketones
i) By alcohols oxidising Typically, primary and secondary alcohols are oxidised to produce aldehydes and ketones, respectively.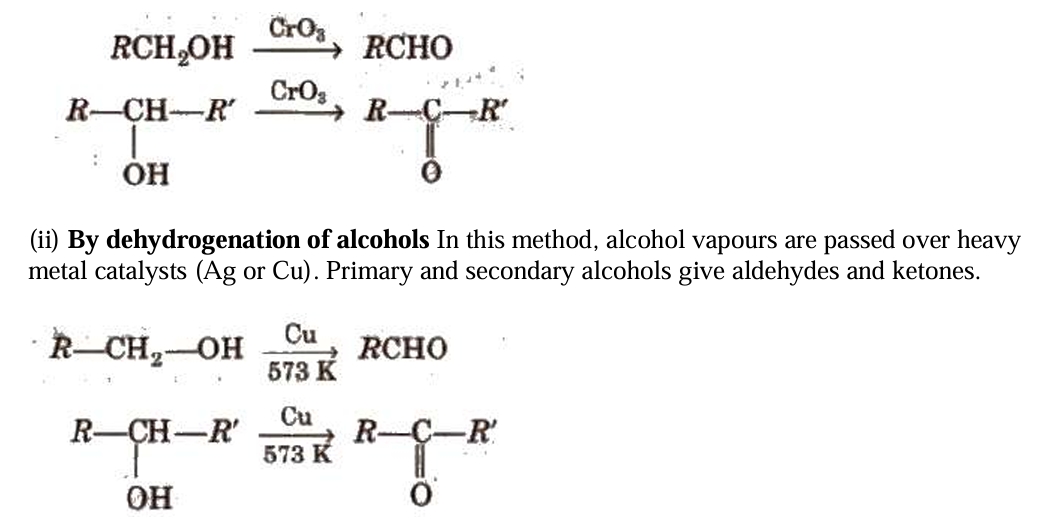
By ozonolysis of alkenes
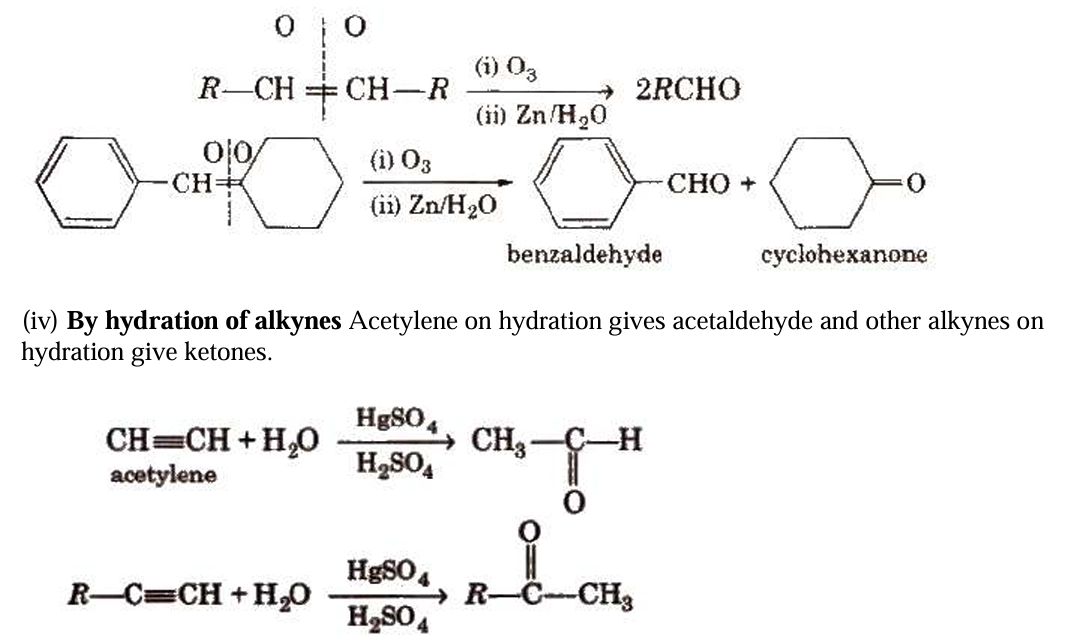
Preparation of Aldehydes
Preparation of Ketones
Physical Properties of Aldehydes and Ketones
1.It is a gas at room temperature, methanal (HCHO). and formalin is the name given to its 40% aqueous solution. It works as a reducing agent to remove colour from vat dyes and silver mirrors. 2. The liquid ethanol (CH3CHO) is volatile. At room temperature, other aldehydes and ketones are either liquid or solid. 3. Because of the strong dipole-dipole interactions, aldehydes and ketones have higher boiling points than ethers and hydrocarbons with similar molecular masses. 4. The boiling points of aldehydes and ketones are lower than those of alcohols with comparable molecular masses because there are no hydrogen bonds between the molecules. 5. Because a hydrogen bond forms with water, the lower members of aldehydes and ketones are miscible with it. However, as the alkyl chain lengthens, the solubility diminishes. 6. Acetophenone is a hypnotic (drug that induces sleep), so it is marketed as hypnone in medicine.Read More - Must-Have CBSE Class 12th Books for 2025 Board Exam
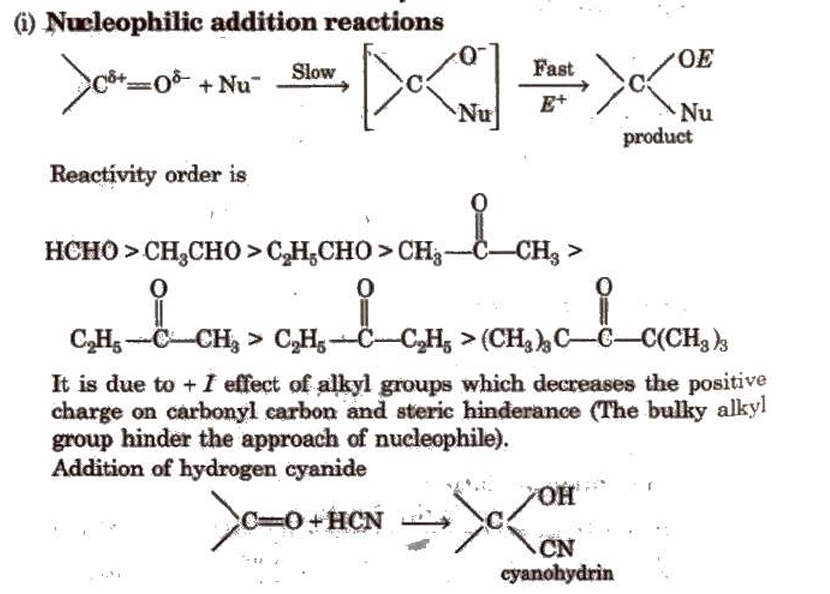
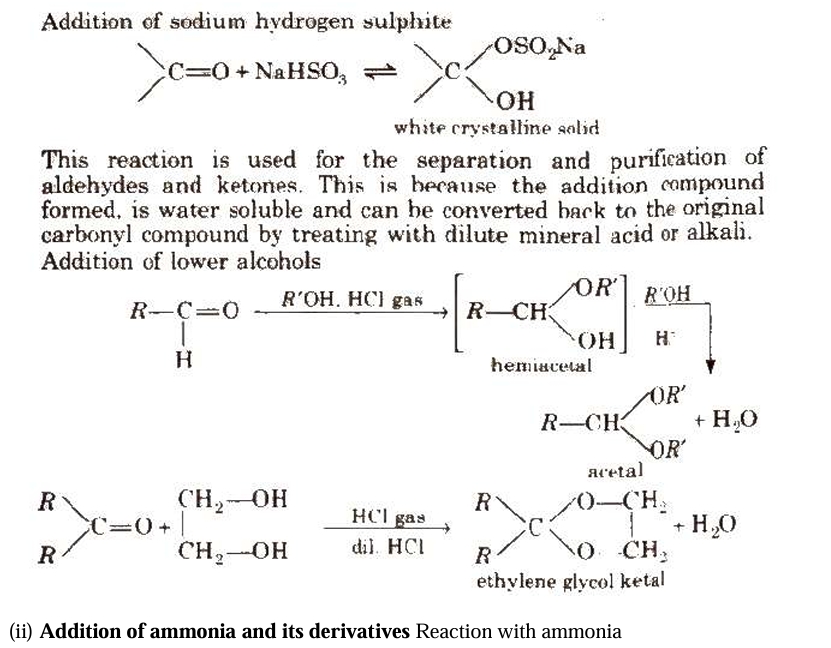
Chemical Reactions of Aldehydes and Ketones
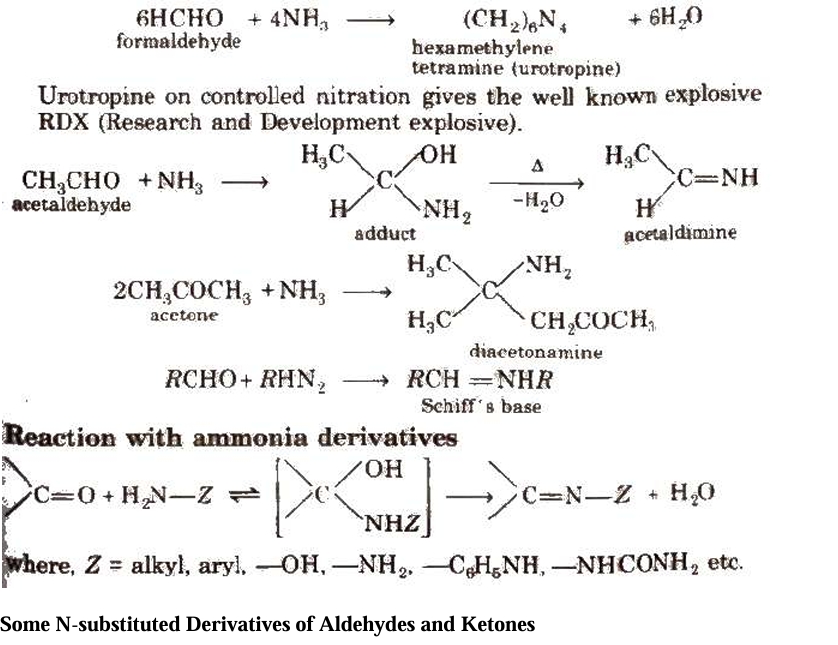
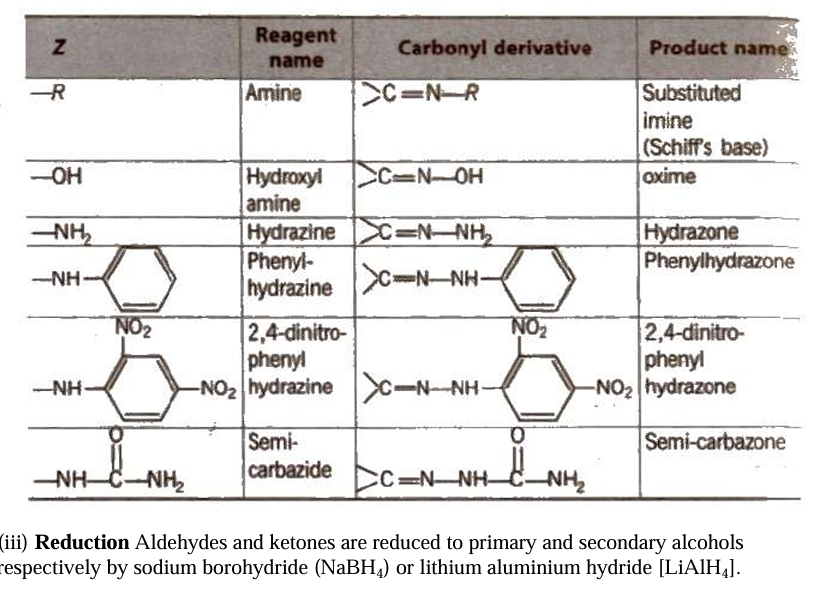
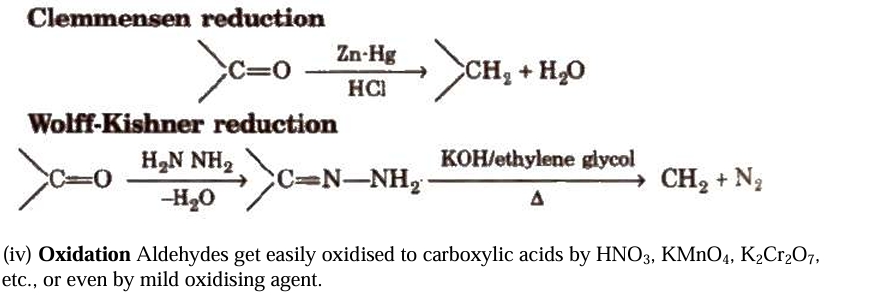
 Fehling solution is made up of a 1:1 mixture of Fehling solutions A and B.
Aqueous copper sulphate is Fehling solution A, while alkaline sodium potassium tartrate, sometimes known as Rochelle salt, is Fehling solution B.
Fehling solution is made up of a 1:1 mixture of Fehling solutions A and B.
Aqueous copper sulphate is Fehling solution A, while alkaline sodium potassium tartrate, sometimes known as Rochelle salt, is Fehling solution B.
(c) Benedict's resolution It also produces red ppt. of CU2O in aldehydes, with the exception of benzaldehyde.
Schiff's reagent -
This is an aqueous solution of magenta or pink-colored rosaniline hydrochloride that has been decolored by passing SO2. Ketones do not react with this reagent to produce a pink hue, but aldehydes do.
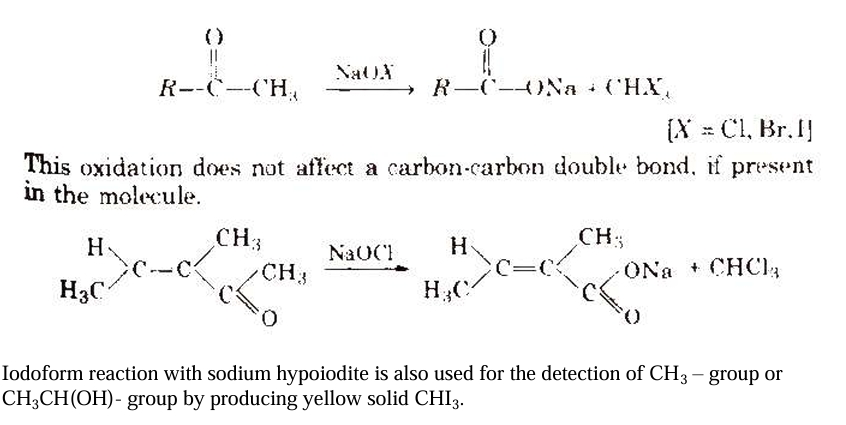
Carboxylic Acids
Carboxylic acids are organic compounds that contain a carboxyl group (-COOH), which is made up of a carbonyl group (C=O) attached to a hydroxyl group (OH). The general formula is R-COOH, where "R" is an alkyl or aryl group.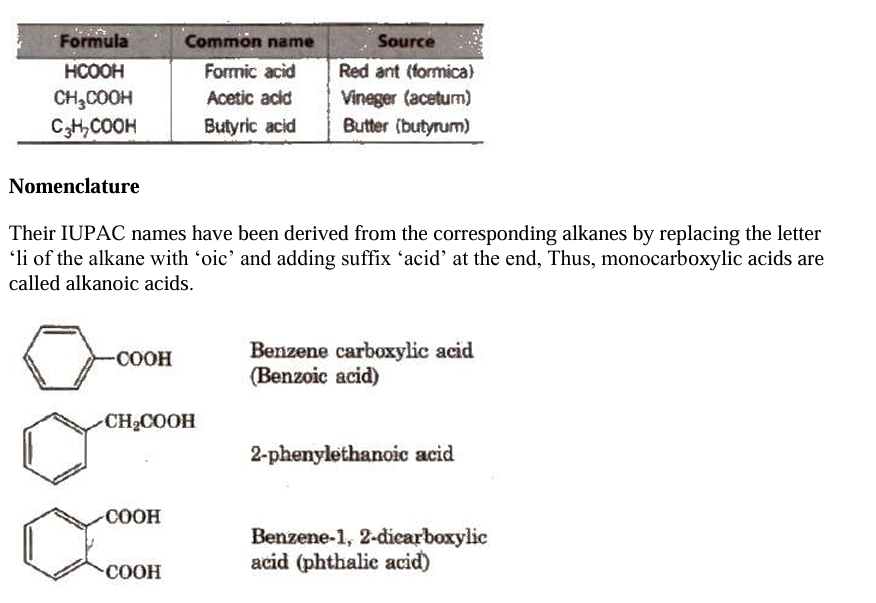
Methods of Preparation of Monocarboxylic Acids
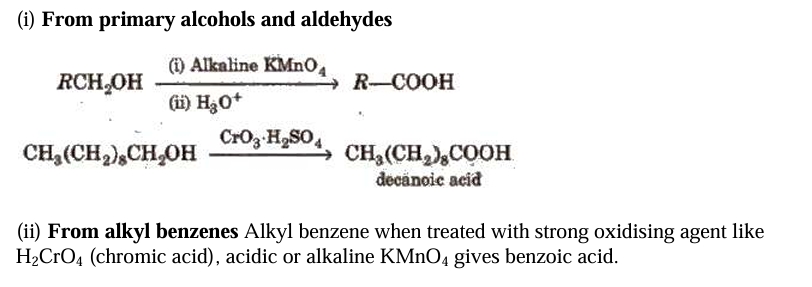
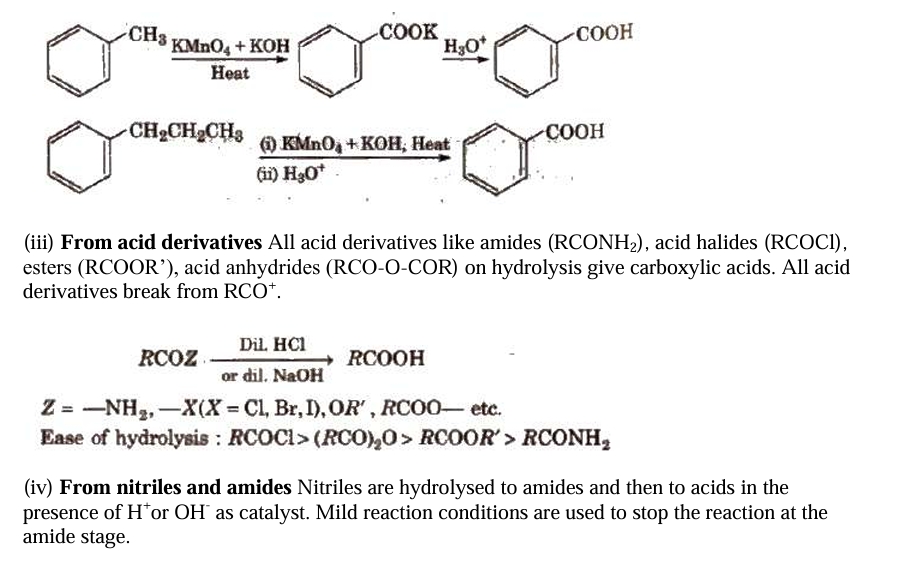
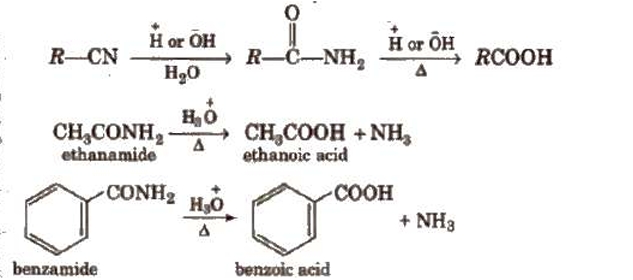 Using Grignard reagents (v) Grignard reagents react with carbon dioxide (dry ice) to produce carboxylic acid salts, which are then acidified with mineral acid to produce matching carboxylic acids.
Using Grignard reagents (v) Grignard reagents react with carbon dioxide (dry ice) to produce carboxylic acid salts, which are then acidified with mineral acid to produce matching carboxylic acids.
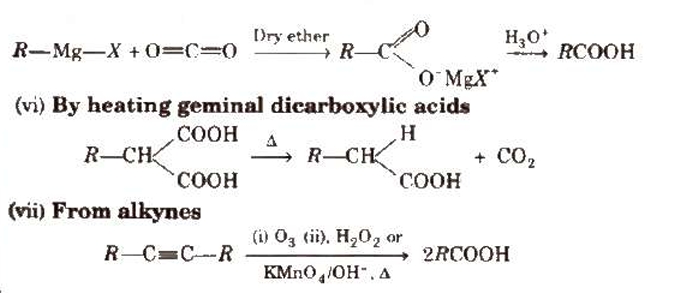
Physical Properties of Carboxylic Acids
1. At normal temperature, aliphatic carboxylic acids with up to nine carbon atoms are colourless liquids with disagreeable odours. Higher acids resemble solid wax. 2. Due to intermolecular hydrogen bonding with H2O molecules, the lower carboxylic acids are easily miscible with water. However, when the alkyl group's size increases, the solubility in water gradually diminishes. 3. Because of their greater intermolecular hydrogen bonding than alcohols with similar molecular weights, monocarboxylic acids have higher boiling temperatures, as the following illustration illustrates.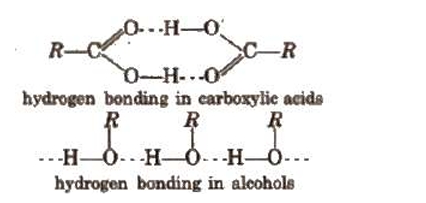 4. Aliphatic monocarboxylic acids' melting points exhibit an oscillation or alternation effect; that is, an acid with an even number of carbon atoms has a greater melting point than its next lower and higher homologue, which has an odd number of carbon atoms. This is due to the fact that the terminal -CH3 and -COOH groups of acids with an even number of carbon atoms are located on opposing sides of the zigzag chain. They consequently become densely packed within the crystal lattice.
5. Glacial acetic acid is the solid form of acetic acid and is entirely pure acetic acid.
Glacial acetic acid is the name given to pure acetic acid that has turned into an ice-like solid below 16.6°C in temperature.
4. Aliphatic monocarboxylic acids' melting points exhibit an oscillation or alternation effect; that is, an acid with an even number of carbon atoms has a greater melting point than its next lower and higher homologue, which has an odd number of carbon atoms. This is due to the fact that the terminal -CH3 and -COOH groups of acids with an even number of carbon atoms are located on opposing sides of the zigzag chain. They consequently become densely packed within the crystal lattice.
5. Glacial acetic acid is the solid form of acetic acid and is entirely pure acetic acid.
Glacial acetic acid is the name given to pure acetic acid that has turned into an ice-like solid below 16.6°C in temperature.
Benefits of CBSE Class 12 Chemistry Notes Chapter 12
The CBSE Class 12 Chemistry Notes for Chapter 12 on Aldehydes, Ketones, and Carboxylic Acids offer several benefits for students:Concise and Organized Content : The notes provide well-structured summaries of key concepts, helping students quickly grasp important topics like nomenclature, structure, and reactions of aldehydes, ketones, and carboxylic acids.
Simplified Explanations : Complex chemical reactions, mechanisms, and properties are explained in a simplified manner, making it easier for students to understand and retain information.
Exam-Focused : These notes highlight important topics frequently asked in exams, such as nucleophilic addition reactions, preparation methods, and industrial applications, helping students focus on exam-relevant material.
Time-Saving : By providing concise summaries of lengthy textbook content, the notes save time during revision and help students cover the entire chapter quickly before exams.
Clarifies Doubts : The notes clear common doubts related to reaction mechanisms, functional groups, and the behavior of these compounds, enabling better concept clarity.
CBSE Class 12 Chemistry Notes Chapter 12 FAQs
What are the important reactions between aldehydes ketones and carboxylic acids?
What is the common name of aldehydes and ketones?
Is CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids helpful?
Can I access CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids offline?










