
CBSE Class 12 Chemistry Notes Chapter 8: CBSE Class 12 Chemistry Notes are important for students studying Science, as Chemistry is a crucial sub-subject that requires properly written answers. Scoring well in Chemistry is just as important as other subjects.
Our notes comprehensively cover key concepts such as the electronic configuration, oxidation states, and complex formation of these elements. Understanding the characteristics of d- and f-block elements is important as they play a significant role in various chemical processes and industrial applications. By thoroughly studying these notes students can grasp the complexities of these elements and enhance their performance in their Chemistry exams.CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements Overview
These notes are prepared by subject experts of Physics Wallah provide an in-depth overview of Chapter 8 The d- and f-Block Elements. They provide a clear and detailed understanding of the properties, trends, and applications of transition metals (d-block) and inner transition metals (f-block). The notes cover important topics such as the characteristic properties of d-block elements, their variable oxidation states, formation of complexes, and their role in catalysis. For the f-block elements the notes elucidate the unique features of lanthanides and actinides, their electronic configurations, and their significance in various technological and industrial fields.CBSE Class 12 Chemistry Notes Chapter 8 PDF Download
For a detailed understanding of Chapter 8 The d- and f-Block Elements you can access the PDF link provided below. This PDF is prepared by subject experts of Physics Wallah includes thorough notes on the properties, trends, and applications of d-block and f-block elements. It covers essential concepts such as variable oxidation states, complex formation, and the significance of lanthanides and actinides. Download the PDF to enhance your study and boost your performance in Chemistry exams.CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements PDF
CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements
Here we have provided CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements-The d-Block Elements
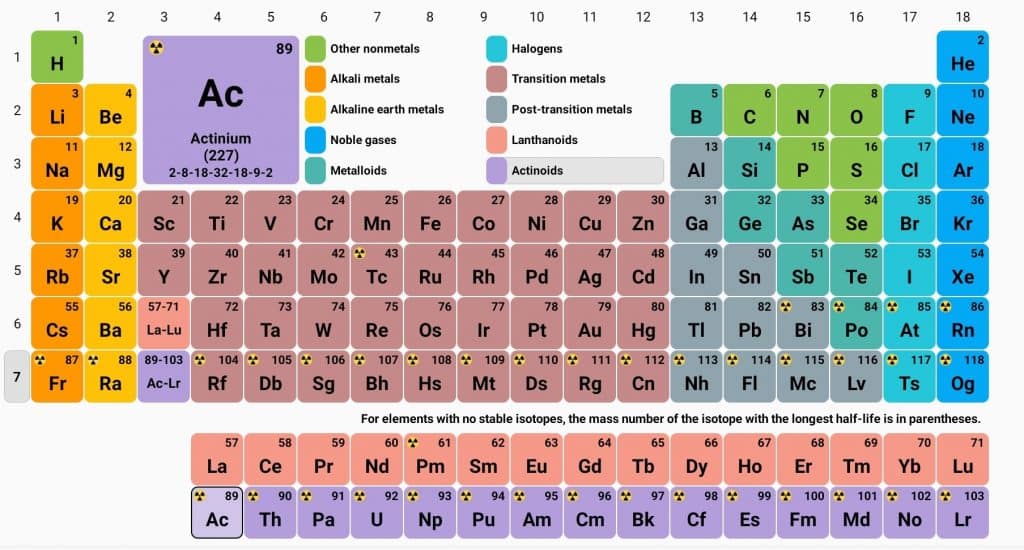 The d-block elements, located in the center of the periodic table in groups 3 to 12, are characterized by their general electronic configuration of
[n−1]d1−10ns0−2[n-1]d^{1-10}ns^{0-2}
, where
(n−1)(n-1)
refers to the penultimate (last but one) shell.
These elements are distinguished by their partially filled d orbitals. Transition elements are defined as those with incompletely filled d orbitals in either their ground state or in one of their oxidation states. Notably, zinc, cadmium, and mercury are excluded from this category as they have completely filled d orbitals.
The d-block elements, located in the center of the periodic table in groups 3 to 12, are characterized by their general electronic configuration of
[n−1]d1−10ns0−2[n-1]d^{1-10}ns^{0-2}
, where
(n−1)(n-1)
refers to the penultimate (last but one) shell.
These elements are distinguished by their partially filled d orbitals. Transition elements are defined as those with incompletely filled d orbitals in either their ground state or in one of their oxidation states. Notably, zinc, cadmium, and mercury are excluded from this category as they have completely filled d orbitals.
The f-Block Elements
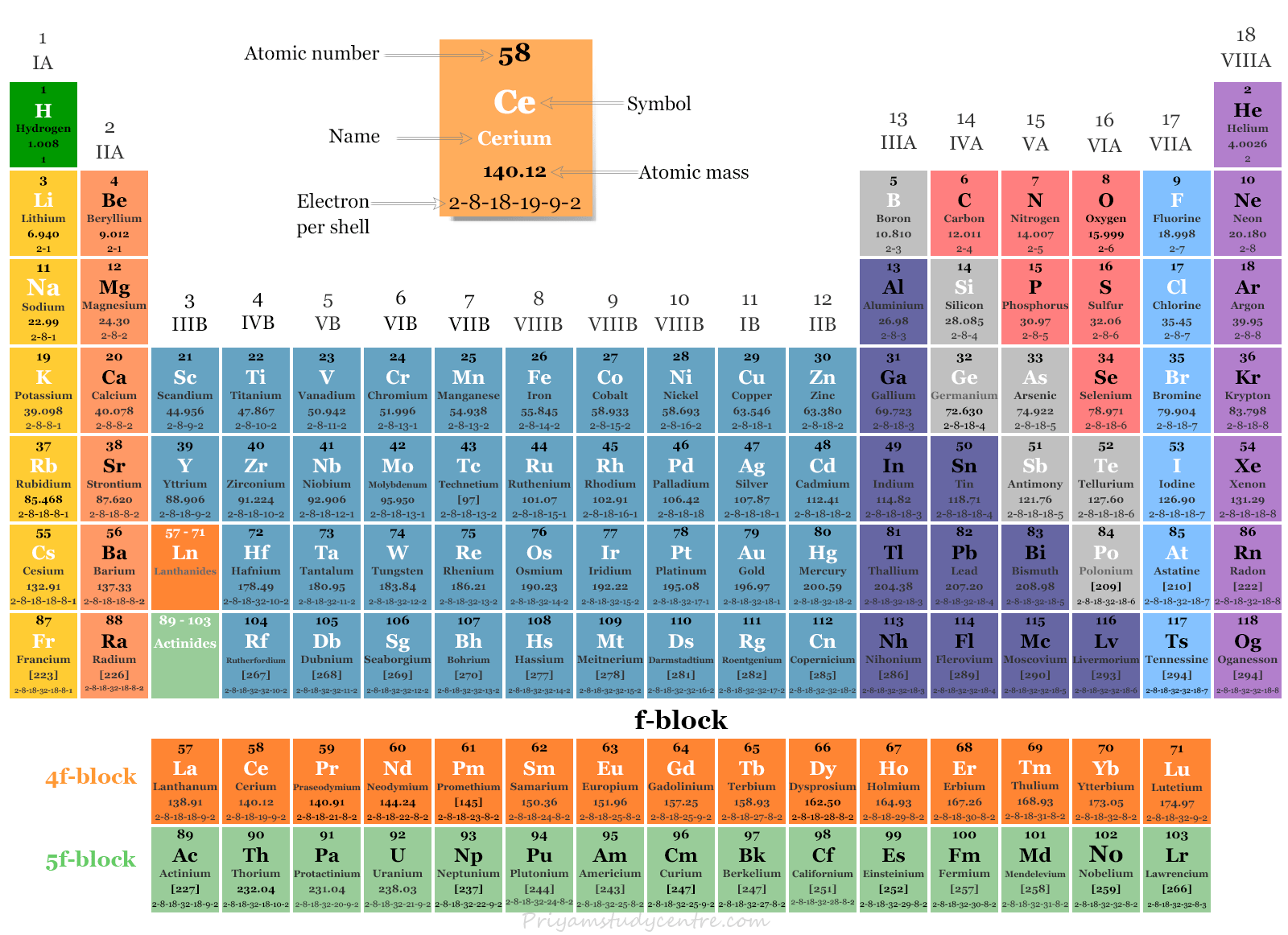 The f-block elements are those in which the 4f and 5f orbitals are progressively filled, and they are situated in the two separate rows below the main body of the periodic table.
The f-block elements are those in which the 4f and 5f orbitals are progressively filled, and they are situated in the two separate rows below the main body of the periodic table.
- Lanthanoids: The lanthanoids consist of 14 elements from Cerium (58) to Lutetium (71), following lanthanum (57). These elements are part of the first inner transition series and exhibit similar properties. Lanthanum, although not a lanthanoid itself, shares similar characteristics and is often studied alongside this series.
- Actinoids: The actinoids include 14 elements ranging from Thorium (90) to Lawrencium (103), following actinium (89). These elements make up the second inner transition series and display similar chemical properties. Actinium, like lanthanum for the lanthanoids, is studied with the actinoids due to its analogous properties.
Four Transition Series
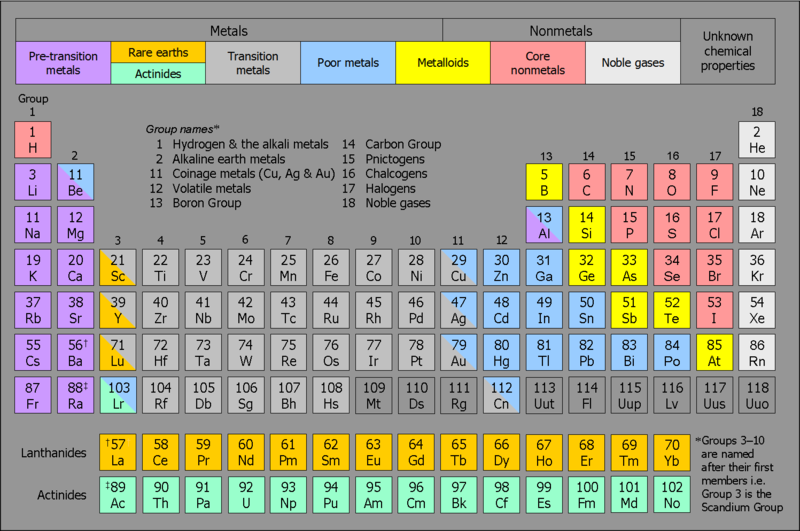 3d-Transition Series:
This series includes transition elements with atomic numbers from 21 (Scandium, Sc) to 30 (Zinc, Zn). These elements have incomplete 3d orbitals and are known for their distinct physical and chemical properties, including variable oxidation states and the ability to form complex compounds.
3d-Transition Series:
This series includes transition elements with atomic numbers from 21 (Scandium, Sc) to 30 (Zinc, Zn). These elements have incomplete 3d orbitals and are known for their distinct physical and chemical properties, including variable oxidation states and the ability to form complex compounds.
4d-Transition Series: The 4d-transition series spans elements with atomic numbers 39 (Yttrium, Y) to 48 (Cadmium, Cd). These elements feature incomplete 4d orbitals. This series is notable for its unique chemical behaviors and applications in various technological fields.
5d-Transition Series: This series consists of elements with atomic numbers 57 (Lanthanum, La), 72 (Hafnium, Hf) to 80 (Mercury, Hg). These elements have incomplete 5d orbitals and are recognized for their complex chemistry and significant roles in both industrial and scientific applications.
6d-Transition Series: The 6d-transition series includes elements with atomic numbers 89 (Actinium, Ac), 104 (Rutherfordium, Rf) to 112 (Uub). These elements are characterized by their incomplete 6d orbitals and are part of the fourth transition series, which features some of the heaviest and least stable elements.
a) Metallic Character:
Transition elements are inherently metallic, characterized by strong metallic bonds due to the presence of unpaired electrons. This results in properties such as high density, significant enthalpies of atomization, and elevated melting and boiling points.b) Atomic Radii:
The atomic radii of transition elements generally decrease from Scandium (Sc) to Chromium (Cr) due to the increase in effective nuclear charge. However, elements like Iron (Fe), Cobalt (Co), and Nickel (Ni) have similar atomic sizes as the increased nuclear charge is offset by the increased shielding effect. Copper (Cu) and Zinc (Zn) exhibit larger atomic sizes due to increased shielding and electron-electron repulsions.c) Lanthanoid Contraction:
Lanthanoid contraction refers to the progressive decrease in atomic and ionic radii of transition metals as the atomic number increases, caused by the filling of 4f orbitals before 5d orbitals. This contraction results in the atomic radii of the second-row transition elements being similar to those of the third-row elements.d) Ionization Enthalpy:
The ionization enthalpies of transition metals show slight and irregular variations due to fluctuating atomic sizes. The ionization energy of the 5d series is higher compared to the 3d and 4d series, attributed to the lanthanoid contraction.e) Oxidation States:
Transition metals exhibit a range of oxidation states because both (n-1)d and ns electrons can participate in bond formation, leading to their variable oxidation states.f) Magnetic Properties:
Most transition metals are paramagnetic due to unpaired electrons, which also contribute to their colorful compounds. Magnetic properties increase from Scandium (Sc) to Chromium (Cr) and then decrease as the number of unpaired electrons changes. They are rarely diamagnetic.g) Catalytic Properties:
Transition metals are effective catalysts because of several factors: the presence of incomplete or empty d-orbitals, large surface areas, variable oxidation states, and their ability to form complexes. Examples include Iron (Fe), Nickel (Ni), Vanadium (V2O3), Platinum (Pt), Molybdenum (Mo), and Cobalt (Co).h) Formation of Colored Compounds:
The transition metals form colored compounds due to the presence of incompletely filled d-orbitals and unpaired electrons, which enable d-d transitions. These elements absorb specific wavelengths of visible light and reflect complementary colors, resulting in their characteristic colors.i) Formation of Complexes:
Transition metals are known for forming complexes because of their vacant d-orbitals of suitable energy, small ionic sizes, and high cationic charges. This property is crucial in various chemical reactions and processes.j) Interstitial Compounds:
Transition metals can form interstitial compounds by accommodating small atoms like Carbon (C), Hydrogen (H), Nitrogen (N), and Boron (B) in the interstitial spaces of their crystal lattices. These compounds are non-stoichiometric, meaning their composition can vary, and they tend to be harder and less malleable and ductile. Examples include steel.k) Alloy Formation:
Transition metals readily form alloys due to their similar ionic sizes, which allow metals to replace each other in the crystal lattice. Common examples of alloys include brass, bronze, and steel.Preparation of Potassium dichromate ( ): It is prepared by fusion of chromate ore (FeCr2O4) with sodium carbonate in excess of air.
Effect of pH on chromate and dichromate ions: The chromates and dichromates are inter-convertible in aqueous solution depending upon pH of the solution. The oxidation state of chromium in chromate and dichromate is the same.
Potassium dichromate acts as a strong oxidizing agent in acidic medium:
Preparation of Potassium permanganate (KMnO4):
a) Potassium permanganate (KMnO₄) is synthesized by fusing manganese dioxide (MnO₂) with an alkali metal hydroxide, such as potassium hydroxide (KOH), in the presence of oxygen (O₂) or an oxidizing agent like potassium nitrate (KNO₃). This process produces potassium manganate (K₂MnO₄), which is dark green in color. In neutral or acidic solutions, potassium manganate undergoes both oxidation and reduction reactions, ultimately converting into potassium permanganate (KMnO₄). This conversion is facilitated by the change in oxidation states of manganese from +6 in manganate to +7 in permanganate, resulting in the characteristic deep purple color of KMnO₄.
b) Commercially, it is prepared by the alkaline oxidative fusion of MnO2 followed by the electrolytic oxidation of manganate (Vl).
c) In laboratory, Mn²+ salt can be oxidized by peroxodisulphate ion to permanganate ion.
In acidic medium: In neutral or faintly basic medium:- Properties of Lanthanoids:
- +3 oxidation state is most common along with +2 and +4.
- Except Promethium, they are non – radioactive.
- The magnetic properties of lanthanoids are less complex than actinoids.
- Properties of Actinoids:
- Actinoids also show higher oxidation states such as +4, +5, +6 and +7.
- They are radioactive.
- The magnetic properties of the actinoids are more complex than those of the lanthanoids.
- They are more reactive.
- Mischmetall
- It is a well-known alloy which consists of a lanthanoid metal and iron and traces of S, C, Ca and Al.
- A good deal of mischmetall is used in Mg-based alloy to produce bullets, shell and lighter flint.
Lanthanoid Contraction
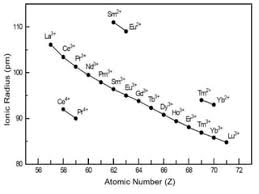 Lanthanoid contraction refers to the progressive decrease in atomic and ionic radii of the lanthanide series elements as the atomic number increases from Lanthanum (La) to Lutetium (Lu). This contraction is due to the ineffective shielding of the increasing nuclear charge by the 4f electrons.
Here's how the oxidation states of some lanthanides are distributed:
Lanthanoid contraction refers to the progressive decrease in atomic and ionic radii of the lanthanide series elements as the atomic number increases from Lanthanum (La) to Lutetium (Lu). This contraction is due to the ineffective shielding of the increasing nuclear charge by the 4f electrons.
Here's how the oxidation states of some lanthanides are distributed:
Ln, Pm, Ho, Er, Lu: These elements primarily exhibit a +3 oxidation state.
Ce, Pr, Tb, Dy: These elements show both +3 and +4 oxidation states.
Sm, Eu, Tm, Yb: These elements can exhibit +2 and +3 oxidation states.
Nd: This element can exhibit +2, +3, and +4 oxidation states.
Benefits of CBSE Class 12 Chemistry Notes Chapter 8 The d-and f-Block Elements
- Comprehensive Coverage: The notes provide a thorough overview of the d- and f-block elements, including their properties, trends, and applications. This ensures that students grasp all essential concepts needed for the exam.
- Clarity and Precision: Expertly prepared notes break down complex topics into clear, concise explanations making it easier for students to understand and retain information.
- Focused Study Material: By concentrating on key topics such as oxidation states, magnetic properties, and catalytic behavior these notes help students focus their study efforts on the most important aspects of the chapter.
- Enhanced Understanding: Detailed explanations and organized content aid in better understanding of the material which is important for tackling exam questions effectively.
- Exam Readiness: By using these notes students can build confidence in their knowledge of the d- and f-block elements, preparing them thoroughly for related exam questions.
CBSE Class 12 Chemistry Notes Chapter 8 FAQs
What are the d-block elements?
What are the f-block elements?
Why are transition elements known for their variable oxidation states?
What is lanthanoid contraction?
How does the magnetic behavior of transition metals arise?










