
CBSE Class 12 Chemistry Notes Chapter 4: In Class 12 Chemistry you will learn advanced concepts that build upon the foundational knowledge gained in previous years. In Class 12 Chemistry the focus shifts towards understanding the intricate details of chemical reactions, properties of elements, and the principles governing them.
These notes are created by subject experts and provide a clear and concise summary of each chapter, ensuring you can easily grasp complex concepts. Whether you are revising for exams or simply want to deepen your understanding, these Chemistry Notes are an important resource throughout your studies.CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics Overview
These CBSE Class 12 Chemistry Notes for Chapter 4 Chemical Kinetics have been prepared by subject experts of Physics Wallah. The notes provide a detailed overview of the chapter focusing on the study of reaction rates and the factors that influence them. You'll find detailed explanations of key concepts such as the rate of reaction, the effect of concentration, temperature, and catalysts, and the significance of the Arrhenius equation.CBSE Class 12 Chemistry Notes Chapter 4 PDF
For a detailed understanding of CBSE Class 12 Chemistry Chapter 4 Chemical Kinetics, you can access the complete notes in PDF format through the link provided below. These notes cover all important topics, including reaction rates, the impact of various factors on these rates, and key concepts like the Arrhenius equation, reaction order, and molecularity.CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics PDF
CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics
Here we have provided CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics-Chemical Kinetics
Chemical Kinetics is a branch of chemistry that focuses on the rate of chemical reactions, the factors influencing these rates, and the mechanisms through which reactions occur. Understanding how quickly a reaction proceeds is crucial, and chemical kinetics provides the framework to analyze this.
Chemical reactions can be categorized based on their rate of reaction:Fast/Instantaneous Reactions: These are reactions that complete in less than 1 picosecond (10⁻¹² seconds). Due to their extremely rapid nature, measuring the speed of such reactions is nearly impossible. Examples include ionic reactions and certain organic substitution reactions.
Slow Reactions: These reactions take a significantly longer time to complete, ranging from minutes to years. Examples include the rusting of iron and the transformation of diamond into graphite.
Moderately Slow Reactions: These reactions fall between the fast and slow categories. They do not complete instantaneously but are faster than slow reactions, making them intermediate in speed.
Rate of Reaction
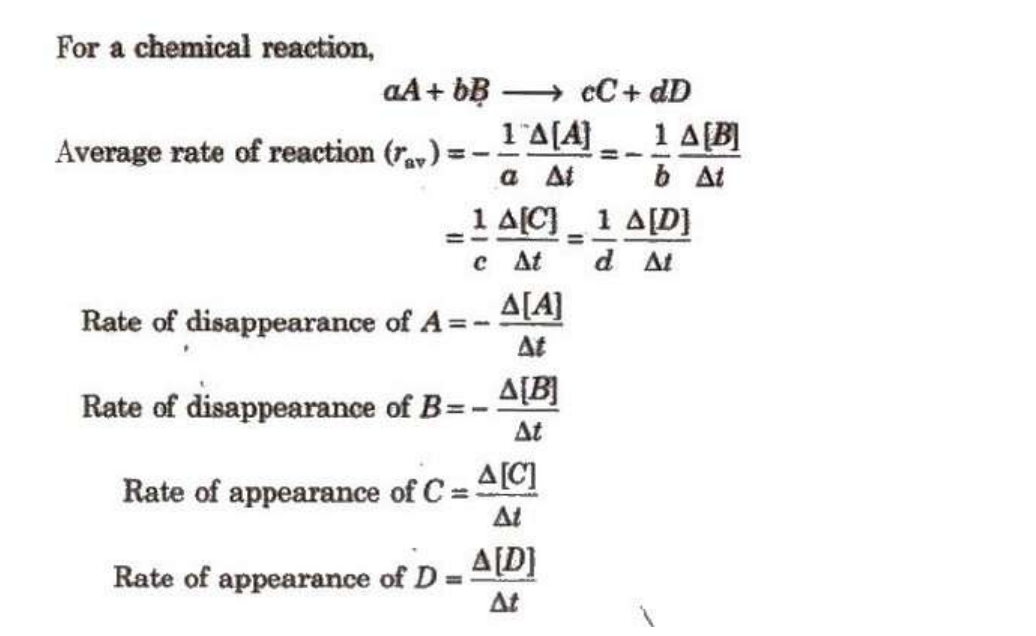
Instantaneous rate of reaction
The instantaneous rate of reaction refers to the rate of a chemical reaction at a specific moment in time. Unlike the average rate, which measures the overall change in concentration over a period, the instantaneous rate provides a snapshot of the reaction rate at a particular point. To measure the reaction rate, several methods can be employed:pH Measurement: Tracking changes in pH can indicate how the concentration of hydrogen ions is varying, which helps in determining the rate of reaction.
Change in Optical Activity: For reactions involving chiral substances, measuring changes in optical rotation can provide information about the rate of reaction.
Change in Pressure: For reactions involving gases, monitoring changes in pressure can be used to determine the rate of reaction.
Change in Conductance: In reactions that involve ionic species, measuring changes in electrical conductance can help assess the reaction rate.
Factors Affecting Rate of Reaction
The rate of a chemical reaction is influenced by several key factors:Nature and Concentration of Reactants: The inherent properties of the reactants and their concentrations play a crucial role. For instance, reactions involving more reactive substances or higher concentrations of reactants typically proceed faster.
Temperature: Increasing the temperature generally accelerates the rate of reaction. This is because higher temperatures provide more energy to the reactant molecules, increasing their collision frequency and the energy available for overcoming the activation energy barrier.
Surface Area of Reactants: The surface area of the reactants affects the reaction rate. A greater surface area allows more reactant molecules to be exposed and interact, speeding up the reaction. For example, powdered solids react faster than larger chunks of the same material.
Radiations and Catalysts: Certain types of radiation can affect reaction rates, especially in photochemical reactions. Catalysts, on the other hand, are substances that speed up a reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy.
Pressure of Gas: For reactions involving gases, increasing the pressure generally increases the rate of reaction. This is because higher pressure increases the concentration of gas molecules, leading to more frequent collisions.
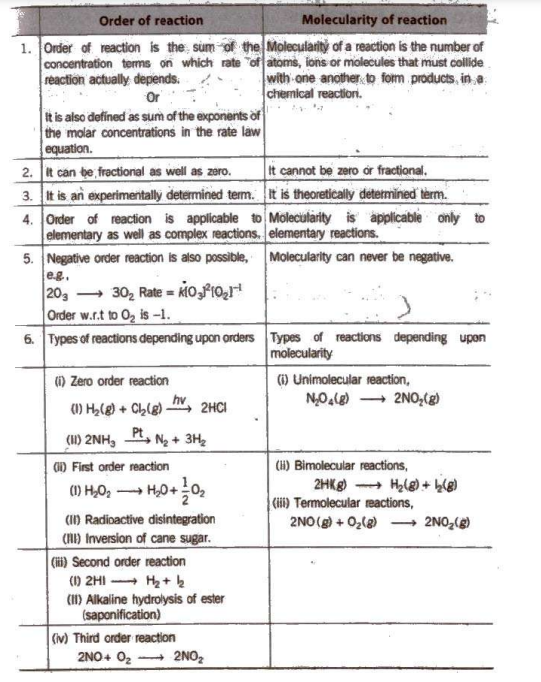
Rate Law Expressions
According to the law of mass action, For a chemical reaction, aA + bB → Products Rate α [A]a [B]b = k[A]a B]b But experimentally, it is observed that the rate of reaction is found to depend upon ‘α’ concentration terms of A and ‘β’ concentration terms of B Then, Rate α [A]α [B]β = k[A]α [B]β where, [A] and [B] molar concentrations of A and B respectively and k is the velocity constant or rate constant. The above expression is known as rate law.Rate Constant
In the above expression, k is called rate constant or velocity constant. Rate constant may be defined as the specific rate of reaction when the molar concentrations of the reactants is taken to be unity, i.e., Rate = k, if [A] = [B] = 1 Units of rate constant or specific reaction rate for a nth order reaction is given as K = (1/Time) x(1/[Conc.]n – 1)Characteristics of rate constant
The rate of a chemical reaction is influenced by several key factors:Nature and Concentration of Reactants: The inherent properties of the reactants and their concentrations play a crucial role. For instance, reactions involving more reactive substances or higher concentrations of reactants typically proceed faster.
Temperature: Increasing the temperature generally accelerates the rate of reaction. This is because higher temperatures provide more energy to the reactant molecules, increasing their collision frequency and the energy available for overcoming the activation energy barrier.
Surface Area of Reactants: The surface area of the reactants affects the reaction rate. A greater surface area allows more reactant molecules to be exposed and interact, speeding up the reaction. For example, powdered solids react faster than larger chunks of the same material.
Radiations and Catalysts: Certain types of radiation can affect reaction rates, especially in photochemical reactions. Catalysts, on the other hand, are substances that speed up a reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy.
Pressure of Gas: For reactions involving gases, increasing the pressure generally increases the rate of reaction. This is because higher pressure increases the concentration of gas molecules, leading to more frequent collisions.
Pseudo First Order Reactions
Pseudo First Order Reactions are chemical reactions that, despite involving more than one reactant, can be treated as first-order reactions under certain conditions. This simplification occurs when one of the reactants is present in such excess that its concentration remains nearly constant throughout the reaction. As a result, the reaction effectively behaves as if it were first-order with respect to the reactant that is not in excess.
In a pseudo first-order reaction, the rate law can be simplified to resemble a first-order reaction. This often happens in reactions where one reactant is present in large excess compared to the other. For example, in the hydrolysis of an ester in the presence of a large amount of water, the concentration of water remains approximately constant, so the reaction rate depends predominantly on the concentration of the ester.Arrhenius Equation
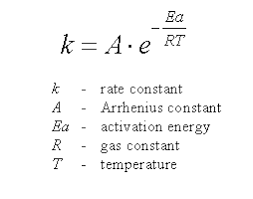 The Arrhenius Equation provides a quantitative relationship between the rate constant of a chemical reaction and the temperature. It helps in understanding how temperature affects the rate of reaction by relating it to the activation energy of the reaction.
The Arrhenius Equation provides a quantitative relationship between the rate constant of a chemical reaction and the temperature. It helps in understanding how temperature affects the rate of reaction by relating it to the activation energy of the reaction.
Activated Complex (or Transition State)
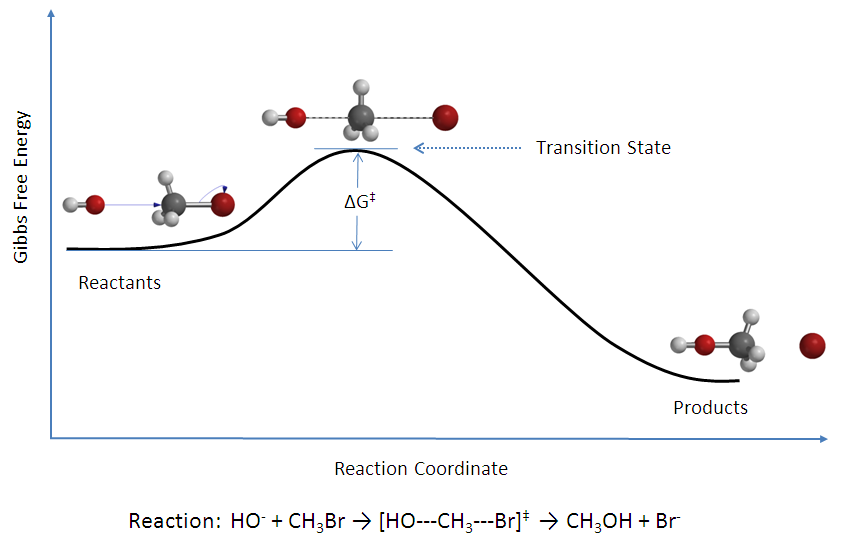 The
activated complex
, also known as the transition state, is a high-energy, unstable intermediate that forms between the reactants and the products during a chemical reaction.
It represents the state where the reactant bonds are partially broken, and the product bonds are partially formed. The activated complex has a very short lifespan and quickly decomposes into the final products or reverts to reactants.
The
activated complex
, also known as the transition state, is a high-energy, unstable intermediate that forms between the reactants and the products during a chemical reaction.
It represents the state where the reactant bonds are partially broken, and the product bonds are partially formed. The activated complex has a very short lifespan and quickly decomposes into the final products or reverts to reactants.
Threshold Energy (E_T)
The threshold energy (E_T) is the minimum amount of energy that reactant molecules must possess to convert into products. This energy must be reached for the reactants to undergo a successful reaction.Activation Energy (E_a)
Activation energy (E_a) is the additional amount of energy required by the reactants to reach the threshold energy and convert into products.
The Activated Complex Theory (or Transition State Theory)
The Activated Complex Theory or Transition State Theory provides a framework for understanding how chemical reactions occur. According to this theory:- Reactants transform into products via an activated complex (or transition state), which is an intermediate state with partially broken and partially formed bonds.
- This theory emphasizes that bond cleavage and bond formation happen simultaneously, and the reactants do not convert directly into products. Instead, they first form a high-energy intermediate state known as the activated complex.
- Energy Barrier: The reactants must overcome an energy barrier to reach the activated complex. The height of this barrier determines the threshold energy required for the reaction to proceed.
Photochemical Reactions
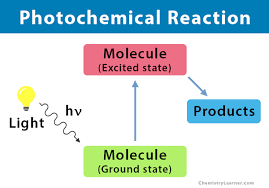
Photochemical reactions are chemical reactions that occur when exposed to visible light or other forms of electromagnetic radiation. Key points include:
- Intensity of Light: The rate of a photochemical reaction is significantly influenced by the intensity of the light. Higher light intensity generally increases the reaction rate by providing more photons to drive the reaction.
- Temperature: Temperature has a relatively minor effect on photochemical reactions compared to other types of chemical reactions. The primary driving force is the light energy rather than thermal energy.
- Quantum Yield (φ): The quantum yield or quantum efficiency of a photochemical reaction measures how effectively light is used in the reaction.
Benefits of CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics
- Comprehensive Understanding: The notes provide a clear and concise explanation of key concepts in chemical kinetics, including rate laws, reaction mechanisms and the Arrhenius equation helping students grasp complex topics effectively.
- Simplified Concepts: Complex ideas such as the activated complex, transition state theory, and factors affecting reaction rates are broken down into simpler terms making them more accessible for students.
- Revision Aid: With concise summaries and key points these notes are an excellent resource for quick revision helping students consolidate their understanding and retain important information.
- Expert Preparation: The notes are prepared by subject experts ensuring that the content is accurate, relevant and aligned with the CBSE curriculum, providing students with reliable study material.
CBSE Class 12 Chemistry Notes Chapter 4 Chemical Kinetics FAQs
What is Chemical Kinetics?
What is the Rate of Reaction?
What is the Arrhenius Equation?
What is Activation Energy (E_a)?
What is the Activated Complex (or Transition State)?










