
CBSE Class 12 Chemistry Notes Chapter 6: Chemistry is a fundamental subject in the CBSE Class 12 curriculum, and mastering it can significantly impact your exam performance. Chapter 6 of the CBSE Class 12 Chemistry syllabus titled General Principles and Processes of Isolation of Elements covers the important methods used to extract metals from their ores and purify them for practical use. This chapter addresses key concepts such as the concentration of ores, various extraction techniques, and the refining processes involved.
By understanding these principles and processes, students can grasp how metals are separated from their natural compounds and refined for industrial applications. This approach will help in achieving a deeper understanding and improve performance in the CBSE Class 12 Chemistry exam.CBSE Class 12 Chemistry Notes Chapter 6 Overview
CBSE Class 12 Chemistry Notes for Chapter 6 General Principles and Processes of Isolation of Elements have been prepared by subject experts of Physics Wallah. These notes provide a detailed overview of the methods and principles involved in extracting and refining metals from their ores. By following these expert-prepared notes students can efficiently grasp the complex concepts and processes involved in metal isolation ensuring better preparation for their CBSE Class 12 Chemistry exams.CBSE Class 12 Chemistry Notes Chapter 6 PDF Download
CBSE Class 12 Chemistry Notes for Chapter 6 General Principles and Processes of Isolation of Elements are available in the PDF format below. The PDF includes detailed explanations, diagrams, and important information that will help in effective study and preparation.CBSE Class 12 Chemistry Notes Chapter 6 PDF
CBSE Class 12 Chemistry Notes Chapter 6 General Principles and Processes of Isolation of Elements
Here we have provided CBSE Class 12 Chemistry Notes Chapter 6 General Principles and Processes of Isolation of Elements-Introduction
The Earth's crust is rich in a variety of elements, with metals comprising about 70% of these elements. Aluminium is the most abundant metal in the Earth's crust, followed by iron. The distribution of different elements in the Earth's crust is as follows: Oxygen (49%), Silicon (26%), Aluminium (7.5%), Iron (4.2%), Calcium (3.2%), Sodium (2.4%), Potassium (2.3%), Magnesium (2.3%), and Hydrogen (1%). Metals can be found in nature in two primary forms: (i) native state, where they are found in their pure, uncombined form, and (ii) combined state, where they are part of various compounds, depending on their chemical reactivity.
Gangue or Matrix
Gangue or matrix refers to the impurities present in ores. These are unwanted materials that are mixed with the desired metal ores and need to be removed to obtain pure metal.Metallurgy
Metallurgy is the comprehensive scientific and technological process used for the extraction of metals from their ores. It involves various techniques to isolate and purify metals, transforming them from their naturally occurring forms into usable materials.Types of Metallurgical Processes
Pyrometallurgy : This process involves extracting metals through high-temperature treatment. Metals such as copper (Cu), iron (Fe), zinc (Zn), and tin (Sn) are commonly extracted using this method. The high temperatures facilitate the chemical reactions needed to separate the metal from its ore.
Hydrometallurgy : In this method, metals are extracted using aqueous solutions. This process is employed for metals like silver (Ag) and gold (Au). The ores are treated with specific chemicals in water, which selectively dissolve the desired metals.
Electrometallurgy : This technique involves extracting metals from molten salt solutions through electrolytic methods. Metals such as sodium (Na), potassium (K), lithium (Li), and calcium (Ca) are extracted using electrolysis, where an electric current is passed through the molten salt to separate the metal.
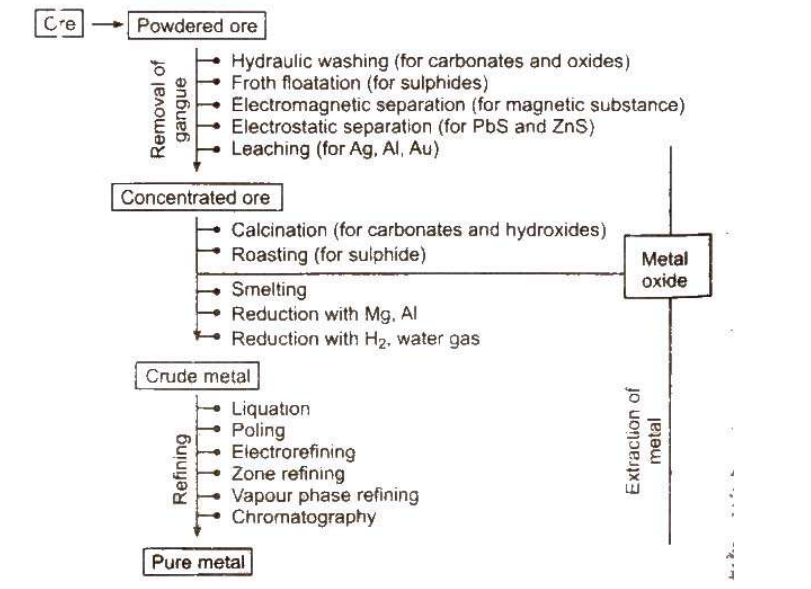
Steps Involved in Metallurgy
The metallurgical process typically involves several key steps, including:- Ore Dressing : The initial step where ores are crushed, ground, and separated from gangue.
- Concentration : The process of increasing the percentage of the desired metal in the ore.
- Reduction : The chemical process to convert the concentrated ore into the pure metal.
- Refining : Purification of the extracted metal to remove any remaining impurities.
Crushing of the Ore
The first step in ore processing involves crushing the large lumps of ore into smaller pieces using jaw crushers. Once crushed, the ore is further ground into a fine powder through pulverization, often using stamp mills. This process increases the surface area of the ore, making it more amenable to further processing.Concentration of Ores
Concentration of ores, also known as ore dressing or beneficiation, involves removing unwanted materials (such as sand and clay) from the ore. This step is important for increasing the concentration of the desired metal in the ore. Various methods are employed depending on the nature of the ore.Hydraulic Washing/Gravity Separation/Levigation
This method separates lighter impurities from heavier ore particles using water. By washing the ore with water, lighter gangue particles are carried away, while the heavier ore particles settle. This technique is based on differences in density and is commonly used for oxide ores like haematite, tin stone, and native ores of gold (Au) and silver (Ag).Froth Floatation
Froth floatation is used to concentrate sulphide ores. This method relies on the differential wetting of ore particles and gangue by water and oil. The ore particles become hydrophobic and float to the surface as froth, while the gangue particles remain in the water. Key agents in this process include:- Stabilisers : Such as aniline or cresols, which stabilize the froth.
- Activators : Like CuSO4, which enhance the floating properties of certain ore components.
- Depressants : Such as NaCN or KCN, which prevent specific particles from floating, aiding in the separation of different minerals.
- Collectors : Including pine oils, xanthates, and fatty acids, which increase the non-wettability of ore particles by water.
Electromagnetic Separation
This method is applied when either the ore or the associated impurities are magnetic. Magnetic ores or impurities, like chromite (FeCr2O4) with magnetic gangue, or wolframite (FeWO4) with non-magnetic impurities, can be separated using electromagnetic separation techniques.Electrostatic Separation
Used for separating conductive and non-conductive materials, this method charges the ore particles in an electrostatic field. For instance, lead sulphide, a good conductor, gets charged and is separated from zinc sulphide, which is a poor conductor and drops vertically from the roller.Chemical Method - Leaching
Leaching involves treating the ore with a chemical reagent that selectively dissolves the ore while leaving impurities behind. For example:- Bauxite is leached with hot concentrated NaOH, which dissolves aluminium, leaving behind other oxides.
- Noble metals like silver (Ag) and gold (Au) are leached with dilute NaCN or KCN solutions in the presence of air, which dissolves the metals while other substances remain undissolved.
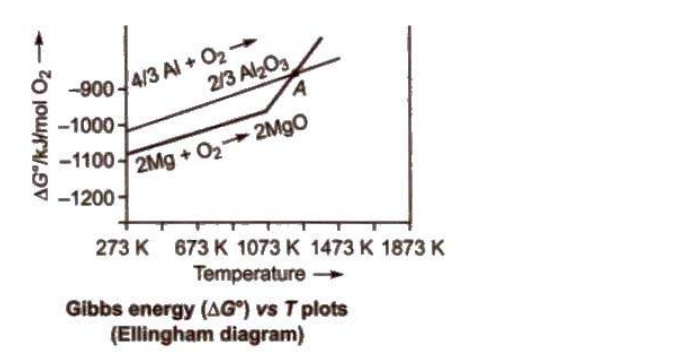
Refining or Purification of Crude Metals
 Once crude metals are extracted from their ores, they often contain various impurities that need to be removed. Refining or purification methods are employed to achieve high-purity metals suitable for industrial use.
Physical Methods
Once crude metals are extracted from their ores, they often contain various impurities that need to be removed. Refining or purification methods are employed to achieve high-purity metals suitable for industrial use.
Physical Methods
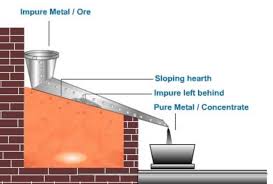
1. Liquation:
- Application: Used for metals with low melting points (e.g., Sn, Pb, Hg, Bi) where the melting point of the metal is lower than that of the impurities.
- Process: The impure metal is placed on a sloping hearth and gently heated. The metal melts and flows down, leaving behind non-fusible impurities.
2. Distillation:
- Application: Suitable for low boiling metals such as zinc (Zn) and mercury (Hg).
- Process: The impure liquid metal is heated to evaporate, and the pure metal is collected as a distillate.
3. Cupellation:
- Application: Used when the impure metal contains impurities that form volatile oxides (e.g., lead in silver).
- Process: The metal is heated in a furnace where impurities are oxidized and removed as volatile oxides, leaving behind the pure metal.
Chemical Methods
1. Poling:
- Application: Used for metals with impurities of their own oxides (e.g., Cu₂O in blister copper, SnO₂ in impure tin).
- Process: The molten impure metal is stirred with green wood poles. The wood liberates gases such as methane (CH₄) that reduce any oxides present in the metal.
2. Electro-Refining:
- Application: Commonly used for metals like copper (Cu), silver (Ag), gold (Au), chromium (Cr), zinc (Zn), and nickel (Ni).
- Process: The impure metal is used as the anode, and a pure metal rod or sheet is used as the cathode. The electrolytic solution contains a soluble salt of the metal. When electricity is passed, the pure metal deposits on the cathode, and insoluble impurities settle as anode mud or sludge.
3. Zone-Refining:
- Application: Used for semiconductors like silicon (Si), germanium (Ge), gallium arsenide (GaAs), and indium antimonide (InSb).
- Process: Based on fractional crystallization, this method exploits the difference in solubility of impurities in the molten and solid states of the metal. It produces elements of very high purity.
4. Vapour Phase Refining:
- Application: Used for metals requiring ultra-pure forms.
-
Methods:
- van Arkel Method: Used for metals like titanium (Ti) and zirconium (Zr) for space technology.
- Mond’s Process: Used for refining nickel (Ni).
5. Chromatographic Method:
- Application: Used for separating and purifying metal ions based on their different rates of adsorption.
- Process: The impure metal is dissolved in a suitable solvent and passed through a column packed with adsorbent (e.g., alumina, Al₂O₃). The metal and impurities are adsorbed at different rates and are then eluted with an appropriate solvent.
Benefits of CBSE Class 12 Chemistry Notes Chapter 6
Clear Understanding: Helps you easily understand how metals are extracted from their ores and the different methods used in the process.
Easy Revision: Summarizes important points and processes, making it quick and simple to review before exams.
Improved Problem-Solving Skills: Includes practice problems that help you get better at solving related chemistry questions.
Visual Aids: Uses diagrams and charts to make complex processes easier to understand.
CBSE Class 12 Chemistry Notes Chapter 6 FAQs
What is metallurgy?
What are gangue or matrix?
What is calcination?
What is roasting?
What is smelting?










