
CBSE Class 12 Chemistry Notes Chapter 14: CBSE Class 12 Chemistry Notes Chapter 14 which includes important biological macromolecules such as carbohydrates, proteins, enzymes, nucleic acids, and vitamins. The chapter discusses the structure, classification, and functions of carbohydrates (monosaccharides, disaccharides, and polysaccharides), proteins (amino acids, peptides), enzymes (biocatalysts and their mechanism of action), and nucleic acids (DNA and RNA, their role in heredity).
It also touches on the role of vitamins in human health and their classification. The chapter emphasizes the biochemical processes essential to life, explaining how these molecules contribute to the structure and function of living organisms.CBSE Class 12 Chemistry Notes Chapter 14 Overview
CBSE Class 12 Chemistry Notes Chapter 14 focuses on the chemistry of molecules essential to biological systems. This chapter introduces carbohydrates, proteins, enzymes, nucleic acids, and vitamins, covering their structures, functions, and classifications.Carbohydrates are classified into monosaccharides (e.g., glucose), disaccharides (e.g., sucrose), and polysaccharides (e.g., starch and cellulose). These molecules are the primary sources of energy in living organisms.
Proteins , composed of amino acids, are vital for building and repairing tissues. The chapter explains the primary, secondary, tertiary, and quaternary structures of proteins, highlighting their biological importance.
Enzymes act as biocatalysts, speeding up biochemical reactions. Their mechanism of action, such as the lock-and-key model and enzyme inhibition, is discussed in detail.
Nucleic acids , including DNA and RNA, are the carriers of genetic information. The structure of these molecules, including the double-helix structure of DNA and its role in heredity, is emphasized.
Vitamins are organic compounds required in small quantities for various metabolic functions, and they are classified as water-soluble (e.g., vitamin C) or fat-soluble (e.g., vitamin D).
CBSE Class 12 Chemistry Notes Chapter 14 PDF Download
Here we have provided CBSE Class 12 Chemistry Notes Chapter 14 Biomolecules pdf for the ease of students. Students can easily download this pdf and access it without internet.CBSE Class 12 Chemistry Notes Chapter 14 PDF
CBSE Class 12 Chemistry Notes Chapter 14 Biomolecules
Here we have provided CBSE Class 12 Chemistry Notes Chapter 14 Biomolecules - Biomolecules are the organic substances that constitute the building blocks of life; they are in charge of the development and upkeep of living systems.
Carbohydrates
Carbohydrates are optically active polyhydroxy aldehydes (aldcses), ketones (ketoses), or chemicals that hydrolyse to produce these units. Because of the sweet flavour of the simpler parts, they are also known as saccharides (Latin Saccharum = sugar). There are three types of carbohydrates, based on how they behave when hydrolysed.Monosaccharides
These cannot hydrolyse into simpler molecules, which could then be further split into hexoses, pentoses, or tetroses based on the quantity of carbon atoms. Another name for these is homopolysaccharides.- Aldotetroses Erythrose, Threose
- Aldopentoses Xylose, Ribose,
- Aldohexoses Glucose, Galactose,
- Ketohexoses Fructose
Oligosaccharides
 Glycosidic bonds are created when two monosaccharides join together; these bonds are often (1, 4) bonds.
The most common sugar found in plants is sucrose, which is also referred to as cane sugar, table sugar, or invert sugar because it hydrolyses to produce an equimolar mixture of glucose and fructose.
Glycosidic bonds are created when two monosaccharides join together; these bonds are often (1, 4) bonds.
The most common sugar found in plants is sucrose, which is also referred to as cane sugar, table sugar, or invert sugar because it hydrolyses to produce an equimolar mixture of glucose and fructose.
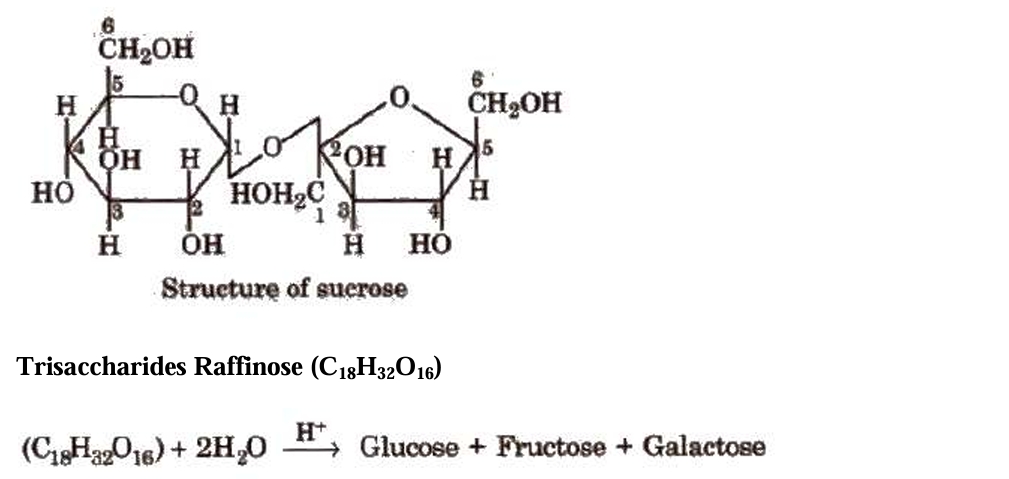
Polysaccharides
These are monosaccharide polymers. Examples include glycogen, cellulose, and starch.Starch
It is a key reserve food in plants and a polymer of glucose. Iodine causes it to turn blue. It consists of two different ingredients: Amylose (20%), an unbranched water soluble polymer. Amylopectin (80%), a branched water insoluble polymer. Sources of starch are potatoes, wheat, rice, maize, etc.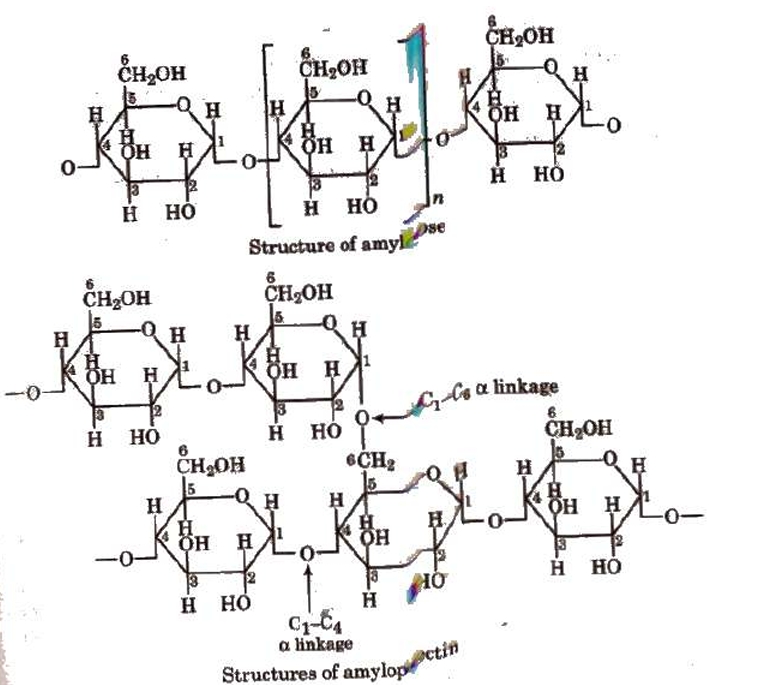
Cellulose
It is the structurally most abundant polysaccharide found in plants. It is a significant source of food for some animals. It is a D (+) β-glucose polymer. The two main sources of cellulose are cotton, which includes 90% of the material, and wood, which contains 50% of the material (the remaining 50% being lignin, resins, etc.).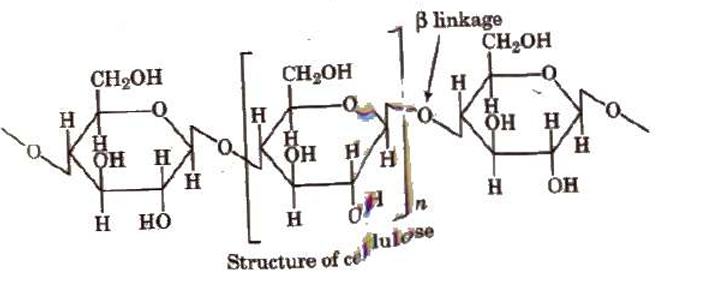 Several materials are obtained from cellulose:
1. Mercerised cotton, to start Cone-treated sodium hydroxide solution gives cellulose a smooth sheen. We refer to it as mercerised cotton.
2. Cotton gunned Blasting gelatin, also known as fully nitrated cellulose (cellulose nitrate), is utilised to make smokeless gunpowder. It is extremely explosive in nature.
3. Acetate of cellulose Motion picture films and acetate rayon are made with it.
4. The polysaccharide It is produced by processing cellulose with carbon disulphide and sodium hydroxide, and it serves as the foundation for VISCOSE rayon.
Several materials are obtained from cellulose:
1. Mercerised cotton, to start Cone-treated sodium hydroxide solution gives cellulose a smooth sheen. We refer to it as mercerised cotton.
2. Cotton gunned Blasting gelatin, also known as fully nitrated cellulose (cellulose nitrate), is utilised to make smokeless gunpowder. It is extremely explosive in nature.
3. Acetate of cellulose Motion picture films and acetate rayon are made with it.
4. The polysaccharide It is produced by processing cellulose with carbon disulphide and sodium hydroxide, and it serves as the foundation for VISCOSE rayon.
Reducing and Non-reducing sugars
Carbohydrates are divided into reducing and non-reducing sugars based on their reducing and non-reducing characteristics. Reducing carbohydrates are defined as those that reduce Fehling's reagent or Tollen's reagent. For example, all mono- and disaccharides (except from sucrose). However, non-reducing carbohydrates are those that do not decrease these reagents. such as carbohydrates and sucrose.Proteins
They are linear polymers of a-amino acids. Structure of Proteins(a) Primary structure
It simply reveals the sequence of amino acids. (a) An additional framework β-pleated sheet structure when R is a small group, or an α-helix shape preserved by hydrogen bonds. (c) The tertiary framework The compact globular shape created by folding and superimposition of polypeptide chains is known as tertiary structure. Covalent, ionic, hydrogen, and disulphide linkages stabilise it. The precise arrangement constitutes the quaternary structure.Classification on the Basis of Hydrolysis Products


Classification on the Basis Functions
1. Structural proteins Fibrous proteins 2. Enzymes Serve as biological catalyst e.g., pepsin, trypsin etc. 3. Hormones Insulin 4. Contractile proteins Found in muscles, e.g., myosin, actin. 5. Antibodies Gamma globulins present in blood. 6. Blood protein Albumms, haemoglobin and fibrinogen. The protein called haemoglobin is globular. Heme is its prosthetic group. It has four polypeptide chains with a total of 574 amino acid units. α-chains refer to the two chains with 141 amino acid residues each, while β-chains are the two chains with 146 amino acid residues. Defective haemoglobin, produced by substituting valine for glutamic acid, is the cause of sickle cell anaemia.Denaturation of Proteins
Denaturation of proteins is the process that modifies the native proteins' three-dimensional structure. A pH shift, the addition of an electrolyte, heating, or the addition of a solvent such as acetone, alcohol, or water can all be the reason.
Enzymes
Enzymes are a class of intricate proteinoids that are made by living things and are responsible for catalysing chemical reactions. Coenzymes are non-proteinous substances that increase the activity of specific enzymes. These include sugar residues, phosphoric acid residues of vitamins like thiamine and riboflavin, heterocyclic ring systems (pyrrole, purine, pyridine, etc.), and metal ions like Mn2+, Mg2+, K+, Na+, Zn2+, Co2+, etc. Exo-enzyme functions outside the cell in which it is synthesised, whereas endo-enzyme acts within the same cell.Nomenclature
Typically, the suffix "ase" is added to the substrate's root name to name them, such as urease, maltase, diastase, invertase, etc.Oxidative Enzymes
They catalyse oxidation-reduction reaction and are mostly conjugated proteins.
Applications of Enzymes
(i) Illness treatment A diet low in phenylalanine can cure the congenital condition phenyl ketonurie, which is brought on by phenylalanine hydroxylase. To stop cardiac disease, blood clotting is accomplished with the enzyme streptokinase. (ii) In the industrial sector, leather tanning, fermentation, etc.Nucleic Acids
Important Terms of Nucleic Acids
1. Nucleotides
Nucleotides consist of 5-carbon sugar + nitrogenous base + 1, 3-phosphate groups.2. Pentose sugar
It is either ribose or deoxy ribose (not having oxygen at C2).3. Nitrogenous base
Derived from purines having two rings in their structure e.g., Adenine (A) and Guanine (G) and derived from pyrimidines having one ring in their structure e.g., Thymine (T), Uracil (U) and Cytosine (C). Two H-bonds are present between A and T (A = T) while three H-bonds are present between C and G (C ≡ G).4. Ribonucleotide
Phosphate unit + Ribose + one base unit from A, G, C, or U.5. Deoxyribose nucleotide
Phosphate unit + Deoxyribose + one base from A, G, C or T6. Nucleoside
Ribose-/deoxyribose + one base unit from A, G, C, Tor U.DNA and RNA
Polynucleotides known as nucleic acids are found in live cells, bacterial cells devoid of a nucleus, and virus-free cells.
Structure of DNA
It is made up of two chains of polynucleotides, each of which forms a right-handed helical spiral that has ten bases in a single spiral turn. The two chains run in opposing directions and coil to form a double helix. The hydrogen bonds between them keep them together.Structure of RNA
It typically takes on a right-handed helical conformation and consists of a single strand of ribonucleotides. An RNA can have up to 12,000 nucleotides in it. In accordance with the conventional base pairing rules—G pairs with C, A couples with U or T—it can base pair with complementary strands of DNA or RNA. Similar to DNA, the paired strands in RNA-RNA or RNA DNA are antiparallel. Heterocyclic base and phosphate ester connections are located at C1 and C5„ of the sugar molecule, respectively, in both DNA and RNA.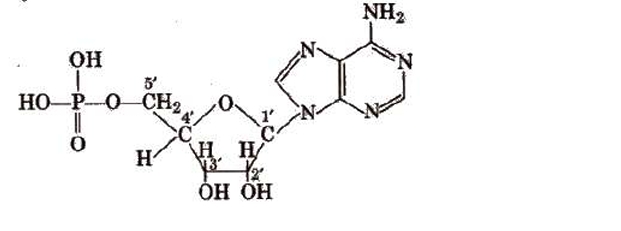
Types of RNA
m-RNA, or messenger RNA It is created in the nucleus and contains instructions for making proteins. RNA transfers (s-RNA, t-RNA) that are soluble or adoptive. It's located in the cytoplasm. Its job is to gather amino acids from the cytoplasm so that proteins can be made.Functions of Nucleic Acids
1. Direct the synthesis of proteins. 2. Transfer the genetic information (hereditary characters).Duplication
It's the method by which a DNA molecule can replicate. Model It denotes a pattern. The parent strand acts as a template for the DNA replication process.Gene - A gene is the section of DNA that contains information about a particular protein.
genetic code Genetic code refers to the relationship between an amino acid and a nucleotide triplet.Codons - In RNA, nucleotide bases work in triplicate groupings (triplets) to code amino acids. Codons are these basic triplets.
The terms "world code," "codon," and "anticodon," respectively, relate to m-RNA, t-RNA, and DNA.Benefits of CBSE Class 12 Chemistry Notes Chapter 14
The CBSE Class 12 Chemistry Chapter 14 "Biomolecules" notes provide several benefits for students:Clear Understanding of Biological Chemistry : The notes help students grasp the chemical composition and role of essential biomolecules like carbohydrates, proteins, enzymes, nucleic acids, and vitamins.
Simplified Concepts : Complex topics like enzyme mechanisms, protein structure, and DNA replication are broken down into simple explanations, making it easier to study and remember.
Examination Preparation : The notes highlight key points, definitions, and important reactions, which are crucial for scoring well in board exams.
Time-Saving Resource : Well-organized notes reduce the time needed for revision by summarizing the chapter effectively, making them an efficient study tool.
Improves Retention : Diagrams, charts, and examples included in the notes aid in visual learning and better retention of concepts.
CBSE Class 12 Chemistry Notes Chapter 14 FAQs
Which biomolecule is an organism's main source of energy?
What biomolecules are proteins?
Which element cannot be found in any biomolecule?
What biomolecule is the most abundant?










