Physical And Chemical Properties of Phenols : Any organic compound that belongs to the phenol family can be identified by its aromatic ring-attached hydroxyl (OH) group to a carbon atom. The most basic member of the family is phenol, also known as carbolic acid or monohydroxybenzene (C 6 H 5 OH). Additionally, the whole family is referred to by the generic term, phenol. Their physical and chemical characteristics are different from those of alcohol. The hydroxyl group is mostly responsible for the physical and chemical characteristics of phenols.
Physical properties of Phenols: Pure phenol has a disinfectant-like smell and is a white, crystalline solid. It must be applied carefully since it instantly results in white blisters on the skin. Frequently, the crystals are discolored and quite wet.
Melting and Boiling Point of Phenols: Comparing the melting and boiling points of phenol and methylbenzene (toluene) is helpful. Both molecules are nearly identical in shape and have the same amount of electrons. This indicates that van der Waals dispersion forces will cause very similar intermolecular attractions.Hydrogen bonding is primarily responsible for the higher values of phenol, though permanent dipole-dipole attractions caused by oxygen's electronegativity also play a role. A hydrogen atom on one oxygen molecule and a lone pair on one of its neighbors' -OH groups can form a hydrogen bond.
|
Melting point ( o C) |
Boiling point ( o C) |
|
|
C 6 H 5 OH |
40-43 |
182 |
|
C 6 H 5 CH 3 |
–95.0 |
111 |
Intermolecular hydrogen bonding in phenols
1. The presence of a hydroxyl group determines how soluble phenol is in water.
2. Intermolecular hydrogen bonding is facilitated by the hydroxyl group present in phenol.
3. As a result, phenol molecules and water form hydrogen bonds, which enable phenol to dissolve in water.
4. On the other hand, the aryl group that is joined to the hydroxyl group is hydrophobic.
5. Therefore, when the size of the aryl group increases, the solubility of phenol decreases.
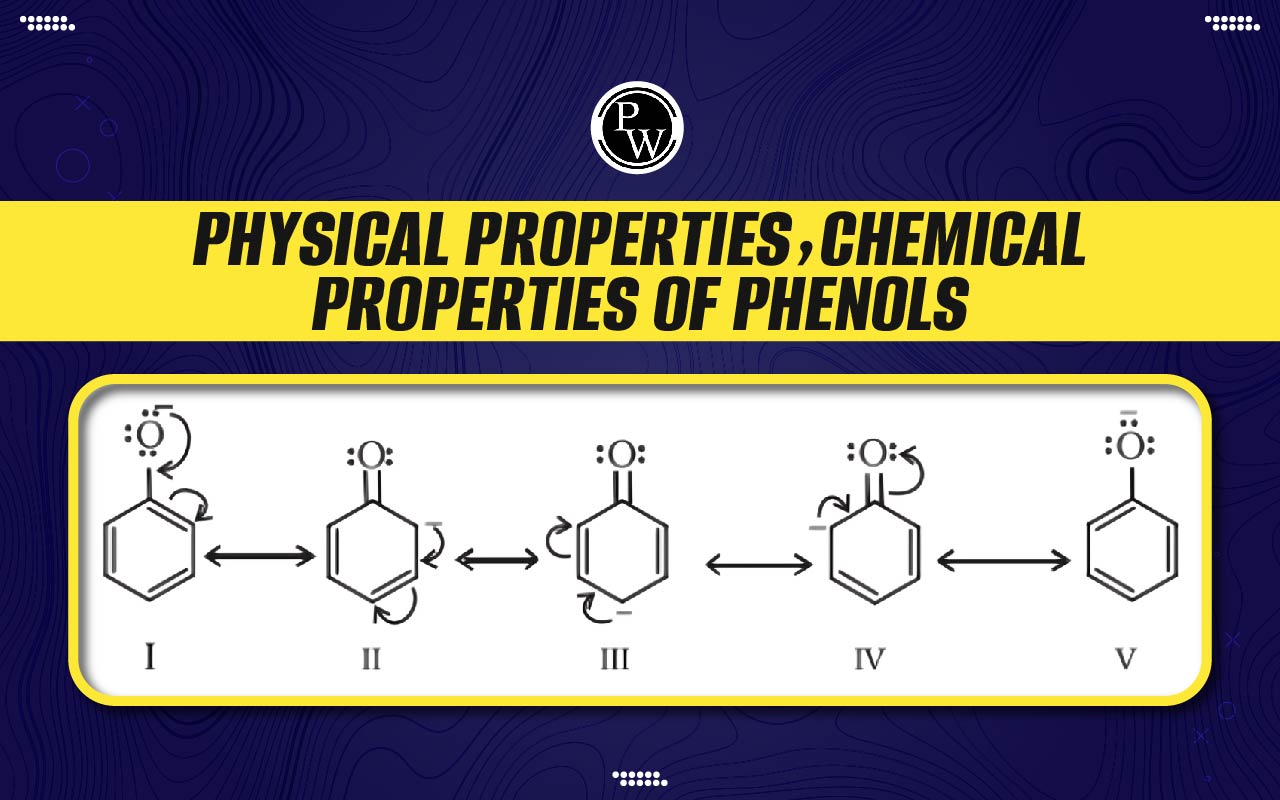
Acidity of Phenols : When phenols interact with active metals like potassium, sodium, etc., the corresponding phenoxide is formed. These phenolic reactions show that it is an acidic substance. The hydroxyl group in phenol is directly connected to the sp 2 hybridized carbon of the benzene ring, which functions as an electron-withdrawing group. As a result, the electron density on oxygen is reduced.
Phenoxide ions exhibit greater stability compared to alkoxide ions because of the delocalization of negative charge within the benzene ring. Phenols are therefore more acidic than alcohols. When it comes to substituted phenols, an electron-donating group attached to the ring results in a decrease in acidity, whereas an electron-withdrawing group results in an increase in acidity.
Chirality in Phenols : A molecule or object is said to be chiral if it is unable to superimpose on its mirror image through translation or relation. This is a property that phenols exhibit in their molecules, particularly in catechin. The lack of axial and two-dimensional symmetry is the cause of this.
Electrophilic substitution reactions : An electrophile can more easily attack the benzene ring due to the increased electron density caused by the presence of an OH group on it. The benzene ring is involved in electrophilic substitution reactions. The ortho and para carbons of benzene have more electrons than the meta position due to the presence of an OH group. The term "o ‒, p ‒ directing group" refers to the OH group.
Kolbe’s reaction: Phenol produces sodium salicylate through a reaction with sodium hydroxide and carbon dioxide, which then acidifies to produce salicylic acid. With an aromatic, electrophilic substitution, the phenoxide particle formed with NaOH is more reactive than phenol, and it thus acidifies to provide hydroxy acid.
Reimer-Tiemann reaction: When phenol reacts with chloroform (CHCl 3 ) in the presence of NaOH, an aryl aldehyde is produced. A group known as -CHO is brought into the ring. This process is referred to as the Reimer-Tiemann reaction. Dichlorocarbene is the name given to the electrophile form that forms in this reaction (CCl 2 ). Salicylaldehyde, the end product, is produced by hydrolyzing intermediate, substituted benzyl chloride in the presence of alkali.
Fries rearrangement: When phenol esters are rearranged in the presence of anhydrous AlCl 3 , phenolic ketones are produced. Fries rearrangement is the term for this reaction.
Acetylation: Acetylation is the process by which phenols react with an acid anhydride and introduce the acetyl radical (CH 3 CO–). Aspirin is the resultant substance.
Nitration: Nitration is the addition of a –NO 2 group to the ring. At 298K, a low temperature, phenol combines with diluted acid to produce ortho and para nitrophenols. Thus, steam distillation is frequently used to separate the ortho and para nitrophenols that are produced. Whereas para nitrophenol has less volatility due to its intramolecular H bonding, ortho nitrophenol is steam volatile due to its intramolecular H bonding. After being treated with concentrated nitric acid, phenol produces two trinitrophenols or acids. The yield is not very good.
Reaction with dilute HNO 3 :
Reaction with Conc. HNO 3 :
Halogenation: Under different experimental conditions, phenols and bromine react to produce entirely different products. Mono bromophenols are produced when it reacts with bromine in low polarity solvents, such as CS 2 or chloroform, at low temperatures. The rationale is that only the ring in positions one and four will be activated by non-polar or low-polar solvents. Therefore, only mono-substituted products are produced.
Bromination is solvents of low polarity like CS 2 :
Reaction with zinc dust: When phenol is distilled with Zn-dust, it gives benzene. It is an example of reduction.
