
CBSE Class 7 Science Notes Chapter 5: "Acids, Bases, and Salts" is one of the most significant chapters in the science syllabus for Class 7. This chapter covers the definitions of acids, bases, and salts as well as their formation in the syllabus. At this point, the students will require the help of the Class 7 Science Chapter 5 notes, which have been developed by our top Chemistry specialists.
These notes will assist them in correctly assimilating the new ideas and applying them to the practice and subsequent test questions. You can use these notes as a resource to review the chapter before an exam, and you can access them from anywhere at any time.CBSE Class 7 Science Notes Chapter 5 Overview
Students in Class 7 may find the chapter daunting at first, but with clear explanations of the topics, studying it will become much easier. You may find the fundamental definitions and explanations of the terminology used in the Acids Bases and Salts Class 7 notes. Experts have appropriately addressed each area of the chapter to enable students to progress methodically and comprehend the material. Let's investigate a little more to learn the purpose of these notes. What acids and bases are will be explained in the Acid, Base, and Salt Class 7 notes. You will have a general understanding of these chemical substances after reading this chapter. You will learn the origins and common usages of the terms "acid" and "base" for universal comprehension. You will be able to recognise and differentiate between these substances by comparing their features and properties. Acids are the non-metallic oxides in aqueous solution that cause the release of hydrogen ions in the mixture. They are sour and present in a wide variety of everyday things. Different acids can be found in fruits, vinegar, curd, and other foods. Conversely, bases are the metallic oxides in an aqueous solution that cause the solution to release hydroxyl ions. Alkalis are bases that dissolve in water. Bitter compounds like soap, washing soda, ammonia, bleach, and so on are examples of bases. The NCERT Class 7 Science Chapter 5 notes, written by the top teachers, can help you gain a deeper understanding of acids and bases.CBSE Class 7 Science Notes Chapter 5 PDF
Here we have provided CBSE Class 7 Science Notes Chapter 5 for the ease of students so that they can just download the pdf and use it easily without the internet. These CBSE Class 7 Science Notes Chapter 5 will help students understand the chapter better.CBSE Class 7 Science Notes Chapter 5 PDF
CBSE Class 7 Science Notes Chapter 5
In Nature, Three Types of Substances are Found:
a. Acids
b. Bases
c. Salts
Acids
Acids have a strong flavour or are naturally acidic. They are quickly rusted. Acid that has been concentrated can rip through clothing and remove wool. It can result in serious burns when the skin is exposed to it. They permit the electric current to flow through them because they are effective electrical conductors. The following lists several acid types: (i) Minerals found in the crust of the planet are used to make mineral acids. (ii) Organic acids, except hydrochloric acid, are those that are created by plants and animals. (iii) Acids that do not entirely dissociate in solution are considered weak acids. Like lactic acid, tartaric acid, etc. Strong acids can fully dissociate in solution (iv). For instance, hydrochloric acid, sulfuric acid, etc.Neutralization
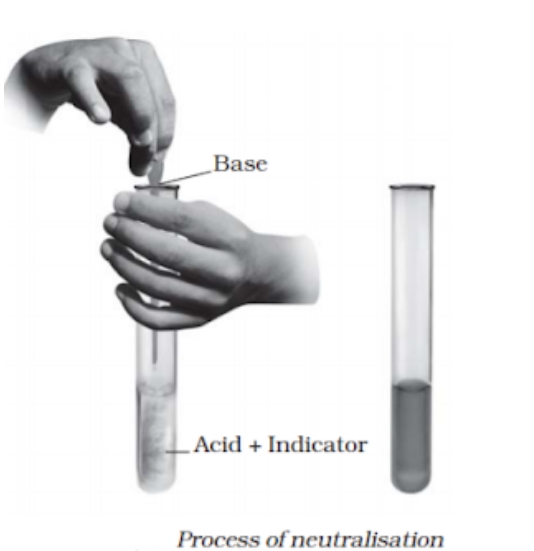 a chemical reaction that produces salt and water when an acid and a base interact. Salt + Base equals Water + Acid. We encounter neutralisation responses daily. Among its instances are:
a. Indigestion: This occurs when the stomach produces excessive amounts of acid. Taking an antacid, such as milk of magnesia, neutralises it and relieves the symptoms.
b. Ant Sting: An ant injects formic acid into the skin through its bite. The injured region can next be treated with calamine, which includes zinc carbonate, or moist baking soda, also known by its chemical name: sodium hydrogen carbonate, to neutralise the ant sting.
c. Soil Treatment: Calcium hydroxide or quicklime (calcium oxide) is added to the soil to neutralise excessively acidic soil.
Ant Sting
Ant sting contains formic acid. The effect of this acid is neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution that contains zinc carbonate.
a chemical reaction that produces salt and water when an acid and a base interact. Salt + Base equals Water + Acid. We encounter neutralisation responses daily. Among its instances are:
a. Indigestion: This occurs when the stomach produces excessive amounts of acid. Taking an antacid, such as milk of magnesia, neutralises it and relieves the symptoms.
b. Ant Sting: An ant injects formic acid into the skin through its bite. The injured region can next be treated with calamine, which includes zinc carbonate, or moist baking soda, also known by its chemical name: sodium hydrogen carbonate, to neutralise the ant sting.
c. Soil Treatment: Calcium hydroxide or quicklime (calcium oxide) is added to the soil to neutralise excessively acidic soil.
Ant Sting
Ant sting contains formic acid. The effect of this acid is neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution that contains zinc carbonate.
Bases
substances with a soapy appearance and a bitter flavour. There are two categories for bases: Weak bases are those that in solution yield fewer hydroxide ions. For instance, the hydroxide of magnesium, the hydroxide of ammonium, etc. Strong bases are those that, when dissolved in water, yield a large number of hydroxide ions. For instance, potassium hydroxide (KOH), sodium hydroxide (NaOH), and so forth. Neutral compounds are defined as those that are neither basic nor acidic. A salt is created when an acid and a base combine to neutralise one another. The nature of salt can be neutral, basic, or acidic.Indicators
Special compounds known as indicators undergo colour changes to signal the presence of other substances. Different hues are displayed by compounds in neutral, basic, and acidic solutions. Because of this, it is frequently used to verify if an acid, base, or neutral solution is present.Classification of Indicators is Given Below:
1. Natural Indicators:
Litmus: Lichens are the source of it. Most commonly, it comes in the form of a solution or thin paper strips. The hue of blue litmus turns red when acid is applied. Red litmus paper becomes blue when bases are added to it. b. Turmeric: This natural indicator stays yellow in acidic and neutral solutions, but turns red when it comes into touch with alkaline ones. c. China Rose: Another naturally occurring indicator that changes basic solutions to green and acidic solutions to dark pink (magenta). d. Red Cabbage: This plant changes basic solutions to blue and acidic solutions to red.Some Other Indicators:
a. Methyl Orange: It produces a pinkish-red colour in acidic solutions and a yellow colour in bases. b. Phenolphthalein: It serves as a gauge for acidity and base. In alkaline liquids, it turns pink, but in acidic solutions, it stays colourless.Benefits of CBSE Class 7 Science Notes Chapter 5
For students getting ready for exams, the Acid Bases and Salts Class 7 NCERT Revision Notes are very important. These revising notes are useful for the following main reasons: Entire Chapter Covered: The review notes offer a thorough rundown of all the key ideas and subjects covered in the Class 7 NCERT Acid Bases and Salts chapter. Concise and Well-Ordered: Students will find it easier to review and remember key topics because the notes provide the material in a clear and well-organized format. Fast Revision: Before exams or tests, students can rapidly review the chapter and review the main points with the aid of these revision notes. Exam-Oriented: The notes assist students in concentrating on important subjects to improve their exam scores by being structured by the format of the test and often asking questions. Clarity and Understanding: The revision notes' straightforward explanations and streamlined language make it easier to learn and absorb difficult subjects. Visual Aids: Adding charts, drawings, and diagrams to the notes helps to visualise the information and improves recall and retention. Time-saving: Students can cover more material more successfully throughout the revision process thanks to the well-structured revision notes. Confidence Booster: Students feel more confident about their exam preparation and are prepared when they use these thorough and trustworthy review notes. Fast Access: Learning is made more flexible and convenient by the easy and quick access to study materials provided by the online revision notes. Supplementary Learning: These notes serve as a helpful tool for independent study and reinforce the material covered in the normal classroom.CBSE Class 7 Science Notes Chapter 5 FAQs
Which is the most important chapter in science class 7?
For CBSE class 7 NCERT books are the base. So, a few chapters are important or may require more effort like acids, bases and salts, soil , respiration in animals, transportation in animals and plants, reproduction in plants, motion and time, light, electric current and its effect.
What is the summary of acid base and salt Class 7?
An acid is defined as a substance whose water solution tastes sour, turns blue litmus red, and neutralizes bases. Base: - A substance is called base if its aqueous solution tastes bitter, turns red litmus blue, or neutralizes acids. Salt: - Salt is a neutral substance whose aqueous solution does not affect litmus.
What is the name of chapter 5 science class 7?
Chapter 5 - Acids, Bases And Salts
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.
Get App







