
Types Of Reagents
GOC of Class 11
Types Of Reagents
There are basically two types of reagents used in organic chemistry, the electrophiles and nucleophiles.
- Electrophiles (Electron−loving)− Electrophilic reagents or electrophiles are the electron−deficient species which tend to attack the substrate at a position (or positions) of high electron density,
e.g. H, H 3 O, NO 2 , NO, PhN 2 , R 2 CH, R, R 3 C, Cl, R-CO, SO3, CO 2 , BF 3 , AlCl 3 , ICI, Br 2 , O 3 etc.
- Nucleophiles (Nucleus−loving)− Nucleophilic reagents or nucleophiles are the electron−rich reagents which tend to attack the substrate at a position (or positions) of low electron density.
e.g. H−,
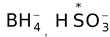 , OH−, RO−, RS−, CN−,
, OH−, RO−, RS−, CN−,
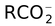 , R − C ≡ C−, −CH(CO
2
Et)2, R−,
, R − C ≡ C−, −CH(CO
2
Et)2, R−,
 , etc.
, etc.
The star indicates the atom that accepts electrons from or donates electrons to the substrate, depending on the case. Electrophiles and nucleophiles in organic reactions can be looked upon essentially as acceptors and donors of electron pairs respectively, from and to other atoms which is mostly carbon.
Also Check




