
Introduction
IUPAC & GOC of Class 12
Nomenclature of Organic Compounds
IUPAC System [International union of pure and applied chemistry]
The most important feature of this system is that any given molecular structure has only one IUPAC name which denotes only one molecular structure.
Salient features of IUPAC system
- A given compound can be assigned only one name.
- A given name can clearly direct in writing of one and only one molecular structure.
- The system can be applied in naming complex organic compounds.
- The system can be applied in naming multifunctional organic compounds.
- This is simple, systematic and scientific method of nomenclature of organic compounds.
Rule for Naming
Prefix (alphabetically) root word (alk) primary suffix (ene, yne) secondary suffix (main functional group)
So IUPAC name of any organic compounds essentially consists of two or three parts.
- Root word
- Suffix
- Prefix
Root Words
The basic unit is a series of root words which indicate linear or continuous chains of carbon atoms. Chains containing one to four carbon atoms are known by special root words while chains from C5 onwards are known by Greek number roots.
| Chain Length | Root word | Chain Length | Root word |
| C 1 | Meth- | C 11 | Undec- |
| C 2 | Eth- | C 12 | Dodec- |
| C 3 | Prop- | C 13 | Tridec- |
| C 4 | But- | C 14 | Tetradec- |
| C 5 | Pent- | C 15 | Pentadec- |
| C 6 | Hex- | C 16 | Hexadec- |
| C 7 | Hept- | C 20 | Eicos- |
| C 8 | Oct- | C 30 | Triacont- |
| C 9 | Non- | C 40 | Tetracont- |
| C 10 | Dec- | C 50 | Pentacont- |
In general, the root word for any carbon chain in alk-.
Primary Suffix
Primary suffix are added to the root words to show saturation or unsaturation in a carbon chain.
| Nature of carbon chain | Primary suffix | Generic name |
| Saturated (C – C) | -ane | Alkane |
|
Unsaturated (C = C)
with one double bond |
-ene | Alkene |
| Unsaturated (C ≡ C) with one triple bond | -yne | Alkyne |
| Unsaturated with two C = C bonds | -diene | Akladiene |
| Unsaturated with two C ≡ C bonds | -diyne | Alkadiyne |
| Unsaturated with three C = C bonds | -triene | Alkatriene |
Secondary Suffix
Suffixes added after the primary suffix to indicate the presence of a particular functional group in the carbon chain are known as secondary suffixes.
| Functional Group | Secondary suffix |
| Alcohol (-OH) | -ol |
| Aldehyde (-CHO) | -al |
| Ketone (>CO) | -one- |
| Carbonxylic acid (-COOH) | -oic acid |
| Sulphonic (-SO3H) | -sulphonic acid |
| Amine (-NH2) | -amine |
| Thioalcohol (-SH) | -thiol |
| Cyanide (-CN) | -nitrile |
| Ester (-COOR) | -oate |
| Amide (-CONH2) | -amide |
| Acid halide (-COX) | -oyl halide |
Note:
The terminal ‘e’ of the primary suffix is removed when initial letter of secondary suffix is vowel. To illustrate the application of above basic rule, the generic names of few classes of organic compounds are given below:
| Homologous series | Root word | Primary suffix | Secondary suffix | Generic name |
| Alcohols (saturated) | Alk | -ane | -ol | Alkanol |
| Alcohols (unsaturated) one double bond | Alk | -ene | -ol | Alkenol |
| Alcohols (unsaturated) one triple bond | Alk | -yne | -ol | Alkynol |
| Aldehydes (saturated) | Alk | -ane | -al | Alkanal |
| Ketones (saturated) | Alk | -ane | -one | Alkanone |
| Carboxylic acids (Saturated) | Alk | -ane | -oic acid | Alkanoic acid |
| Acid chlorindes (saturated) | Alk | -ane | -oyl chloride | Alkanoyl chloride |
Prefix
It should always be kept in mind that alkyl groups forming branches of the parent chain are considered as side – chains. Atoms of groups of atoms such as fluoro (-F), chloro (-Cl), bromo
(-Br), iodo (-I), nitro (-NO
2
), nitroso (-NO) and alkoxy (-OR) are referred to as substituents. Roots words are prefixed with the name of the substituent or side chain.
Arrangement of Prefixes, Root word and Suffixes
These are arranged as follows while writing the name.
Prefix (es) + Root word + Primary suffix + Secondary suffix
For Example:
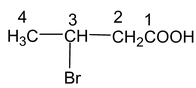
Prefix = Bromo (at position 3),
Root word = But,
Primary suffix = -ane
Secondary suffix = -oic acid
Hence, the name of the compound is,
3 – Bromo butanoic acid
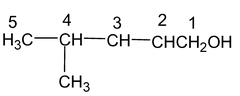
Prefix = Methyl (at position 4)
Root word = Pent,
Primary suffix = -ene (at position 2),
Secondary suffix = -ol
Hence, the name of the compound is,
4 – Methyl pent – 2 – en – 1 – ol
The names of simple aliphatic organic compounds containing only straight chains atoms of various homologous series are described in table as to explain the basic rules of IUPAC system. In case of compounds. Other than hydrocarbons, only the saturated compounds have been considered.
Also Check









