Mechanism Of Organic Reaction
IUPAC & GOC of Class 12
A chemical equation is only a symbolic representation of chemical reaction which indicates the initial reactants and final products involved in a chemical change. Reactants generally consist of two species.
(a) Substrate: One which is being attacked in a chemical reaction
(b) Reagents: The species which attack the substrate molecule
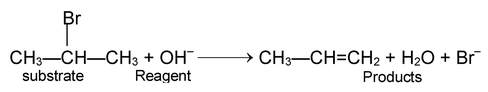
Substrate + Reagent → Products
It is important to know not only what happens in a chemical reaction but also how it happens. Most of the reactions are complex and take place via reactive intermediates which may be or may not be isolated. The reaction intermediates are generally very reactive which readily react with other species present in the environment to form the products. The detailed step by step description of chemical reaction is called its mechanism. Mechanism is only a hypothesis to explain various facts regarding a chemical reaction.
Substrate → Reactive intermediates –— Products
By knowing the mechanism we can predict the product of a chemical reaction, adjust the experimental conditions to improve the yield of the products or even alter the course of reaction to get the different products.
Most of the attacking reagents carry either positive charge (an electron deficient species) or a negative charge (electron rich species). The positively charged reagents attack the substrate at points of high electron density while (-vely) charged reagents attack the point of low electron density. The organic reactions essentially involve changes in the existing covalent bonds present in the molecules. These changes may involve electronic displacements in covalent bonds breaking of some of the existing bonds (bond fission), formation of new bonds as well as energy change accompanying the bond fission and bond cleavage.
We can understand the mechanism of various organic reactions in terms of following well established basic concepts.
(i) Electronic displacement in covalent bond
(ii) Fission (cleavage) of covalent bonds
(iii) Nature of attacking reagents
Types of Organic Reactions:
All organic reactions can be broadly classified into four catagories.
(a) Substitution reactions
(b) Addition reactions
(c) Elimination reactions and
(d) Rearrangement reactions
(a) SUBSTITUTION REACTIONS
In these an atom or a group of atoms in an organic molecule is replaced by another atom or group of atoms without any change in the remaining part of the molecule. These reactions may be initiated by free radical, electrophile or nucleophile.
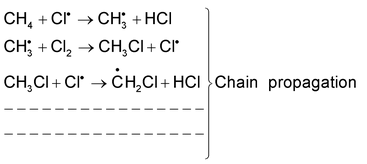
(i) Free radical substitution reaction:
This substitution reaction is brought about by free radicals. For example chlorination of methane in presence of diffused sunlight. The mechanism of the reaction is as follows.
Cl : Cl → 2Cl∙ Chain initiation
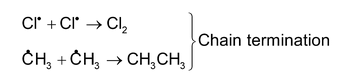
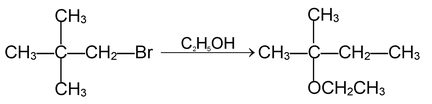
(ii) Nucleophilic substitution reoactions:
These reactions are brought about by nucleophile. The reaction can proceed either via SN 1 or SN 2 mechanism.
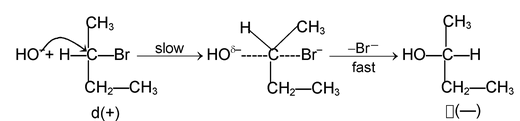
SN 1 mechanism:
Rate determining step involves only the species. For example the reaction.
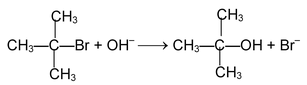
takes place as follows
1
st
step:

2 nd step: Attack by nucleophile
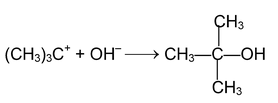
The stability of carbocation is the controlling factor for this mechanism the formation of 3° carbocation as an intermediate proceeds via this mechanism. In an optically active compound substitution at chiral centre through SN 1 mechanism produces recemic mixture (No 100% recemization is observed?).
SN 2 mechanism: Rate determining step involves two species and reaction proceeds through transition state.
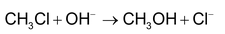
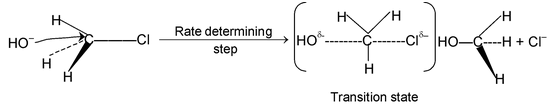
Since 1° carbocation is less stable than the transition state formed above, the reaction involving 1° alkyl halides proceed via SN 2 mechanism. During reaction configuration of carbon is inverted which is referred to as Walden inversion.
Points to Remember
- The higher the polarity of solvent greater the tendency for SN1 reaction.
- High concentration of the nucleophile favours SN2 reaction while low concentration favours SN1 reaction.
- Rearrangement of the carbocation (formed in SN1 reaction) leading to more stable carbocation is observed in SN1 reaction (discussed latter).
- In general SN 2 mechanism is strongly inhibited by increasing steric bulk of the reagents. In such case SN1 mechanism is favoured.
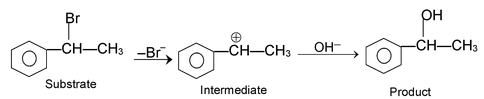
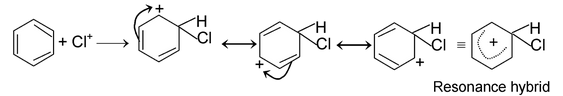
(iii) Electrophilic substitution reactions:
The reaction initiated by an electrophile is known as electrophilic substitution reaction. Aromatic substitution reactions are the examples of this type of reaction.
C
6
H
6
+ Cl
2
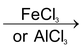 C
6
H
5
Cl + HCl
C
6
H
5
Cl + HCl
The mechanism of this reaction as follows.
Formation of electrophile: Cl : Cl + AlCl
3
⎯→ Cl+

Electrophile attack:
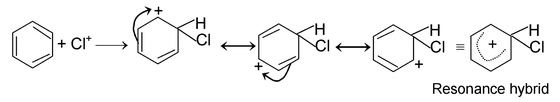
Elimination of proton:
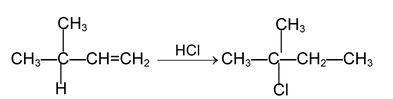
(b) ADDITION REACTIONS
Reactions which involve combination between two molecules to give a single molecule of the product are called addition reactions. Such reactions are typical of compounds containing multiple (double or tripe) bonds. Depending upon the nature of the attacking species (electrophiles, nucleophiles or free radicals) addition reactions are of the following types.
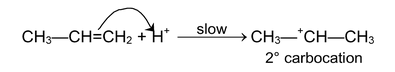
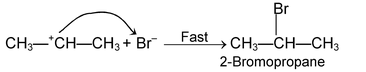
(i) Electrophilic addition reaction:
These reactions are brought about by electrophiles and are typical reactions of alkenes and alkynes.
(ii) Nucleophilic addition reactions:
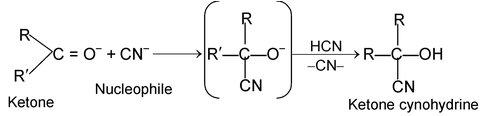
These reactions are brought about by nucleophiles. The characteristics reaction of aldehyde and ketone are nucleophilic addition reaction i.e., base catalysed addition of HCN to aldehydes or ketones.
HO– + HCN ⎯⎯→ H2O + CN–
(iii) Free radical addition reactions:
Addition reactions brought about by free radicals are called free radical addition reactions for example addition of HBr to alkenes in presence of peroxides.
CH
3
—CH=CH
2
+HBr
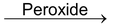
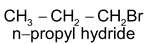
The reaction proceeds through following mechanism.
R - O - O -R
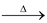 2RO°
2RO°
RO∙ + HBr ⎯⎯→ ROH + Br∙
CH
3
—CH=CH
2
+ Br
 CH
3
—∙CH —CH
2
—Br
CH
3
—∙CH —CH
2
—Br
CH
3
—∙CH—CH
2
—Br
 CH
3
—CH
2
CH
2
—Br + Br
CH
3
—CH
2
CH
2
—Br + Br
Br∙ + Br∙ ⎯⎯→ Br 2
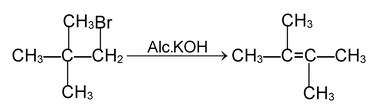
(c) ELIMINATION REACTIONS
An elimination reaction is one which involves the loss of two atoms or groups of atoms from the same or adjacent atoms of a substrate molecule leading to formation of multiple (double or triple) bond. These are of two types.
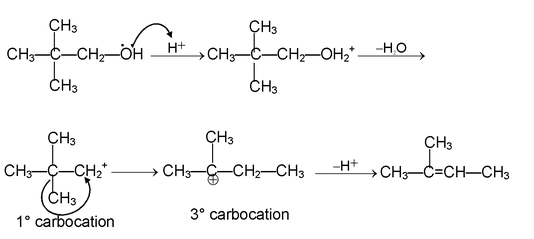
(i) β - elimination reactions. In these reactions, loss of two atoms or groups occurs from the adjacent atoms of the substrate molecule e.g., acid catalysed dehydration of alcohol and base catalysed dehydrogenation of alkyl halides.
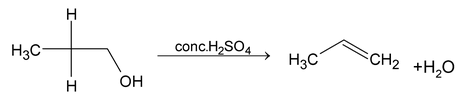
Mechanism:
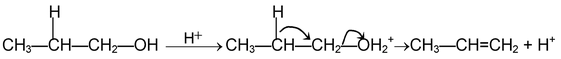
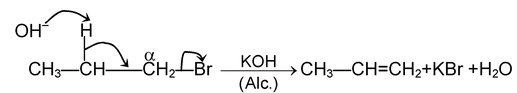
E 1 mechanism
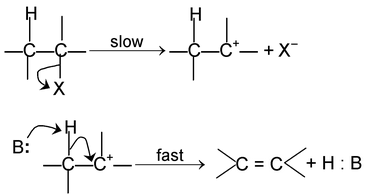
E 2 Mechanism
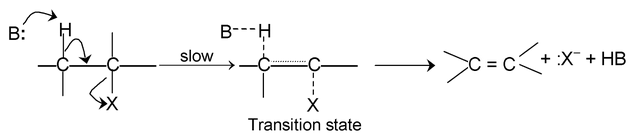
E 1 — CB mechanism
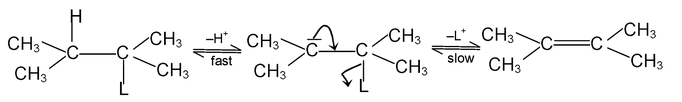
(ii) α - Elimination – In these reactions loss of two atoms or groups occurs from the same atom of the substrate molecule. E.g., base catalysed dehydrohalogenation of chloroform to form dichlorocarbene.
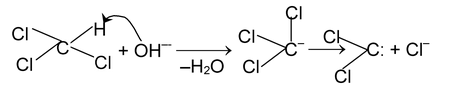
Dichlorocarbene is the reactive intermediate involved in carbylamine reaction and
Reimer – Tieman reaction.
(d) REARRANGEMENT REACTION
These reactions involve the migration of an atom or a group of atoms from one atom to another within the same molecule.
Some reactions involving rearrangement.
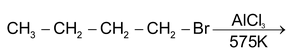
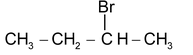
1, 2 Hydride shift
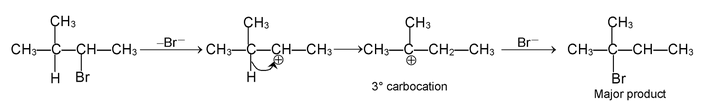
1, 2 Methyl shift