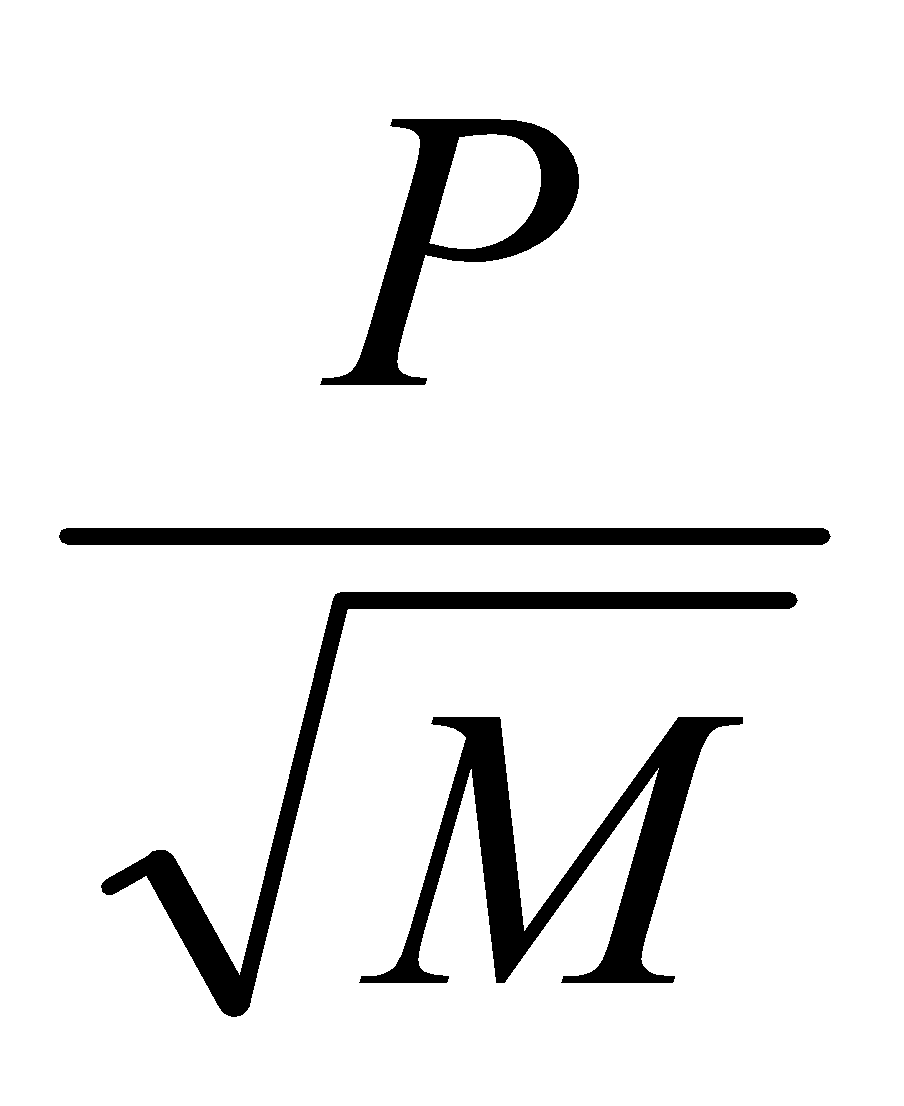STATES OF MATTER
Matter is our surrounding of Class 9
STATES OF MATTER
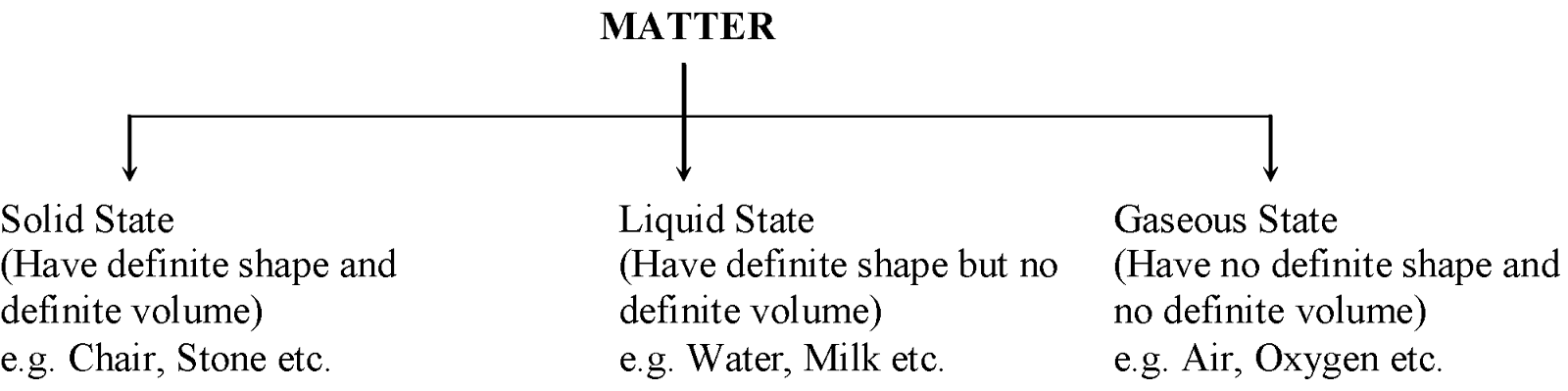
The matter around us exists in three physical states on the basis of physical properties.
- Water exists as ice (solid state), as liquid (liquid state) and as steam (gaseous state).
- Bones and teeth are solids, the blood that flows in our veins is a liquid and the air that we breathe in is a gas.
|
|
The three physical states of matter (i.e solid, liquid or gaseous) arise due to variation in the characteristics of the particles of matter. |
Solids:
A solid is that state of matter which has definite shape, mass and volume.
e.g. : Ice, wood, coal, iron etc.
Properties of Solids:
(a) Solids have a definite shape and definite volume (distinct boundaries): The solids have a fixed shape and distinct boundaries due to small inter particle distances or spaces and strong iner molecular forces of attraction. e.g. when a pen is put in different containers, it does not change its shape.
However, when sugar and salt, are placed in different containers, they take up the shape of the containers, yet they (sugar & salt) are solids. This is because, the shape of individual sugar or salt crystal remains fixed whether we take it in our hand, or put in a jar or in plate.
|
|
The highly ordered arrangement of constituent particles of a solid is called a lattice. This gives rise to a regular geometrical shape to the crystals and define by baris |
(b) Solids possess rigidity: The solids have the tendency to maintain shape, when some outside force is applied (known as rigidity). They may break when dropped or hammered.
However some solids like rubber band, changes its shape when stretched under influence of force, but it regains its original position, when force is withdrawn. However, if excessive force is applied, rubber band breaks.
(c) Solids have a definite volume : Solids have a definite volume as they can not be compressed due to small inter particle distances.
However some solids like sponge can be easily compressed. This is because sponge has minute holes in which air is trapped so that when we press it, air is expelled and the sponge is compressed.
(d) Solids do not possess the property of diffusion : The solids do not have the property of diffusion into other solids (i.e. the particles of two solids do not intermix). This is because the particles of solid do not move much from their positions due to small inter particle distances and strong forces of attraction.
However, particles of some solids like chalk have diffused into other solids like blackboard. i.e. if we write something on blackboard with the chalk and leave it uncleaned for some time, we will find that it becomes difficult to clean the board. This is because of diffusion of chalk particles in between the particles of blackboard and hence it becomes difficult to rub them off.
(b) Liquids :
A liquid is a state of matter which has definite mass and volume but no definite shape.
e.g. : Water, alcohol, milk, mercury etc.
Properties of Liquids
- Liquid do not have fixed shape but have a fixed volume : The liquids have a fixed volume due to strong inter particle forces of attraction in them which are strong to keep the particles together.But these forces are not strong enough to keep the particles in fixed position, therefore, liquids do not have a fixed shape, they take up the shape of vessel in which they are placed.
- Liquids are not rigid but have a property to flow (fluidity): Liquids can flow and change shapes due to larger inter particle distances and weaker forces of attraction in them, than solids. Thus, liquids are not rigid but they possess fluidity (i.e. they have property to flow).
|
|
Relative fluidity of liquids differs from one liquid to other. e.g. water flows faster than honey and it depends on the intermolecular force of attraction between molecule. |
Liquids possess the property of Diffusion : Due to larger inter particle distances in liquids than in solids, the particles of a liquid have more freedom of motion than solids. Thus solids, liquids and gases all can diffuse into liquids as discussed below:
- (i) Diffusion of solids into liquids: When a crystal of copper sulphate or potassium permanganate (solid) is added to water (liquid), the particles of CuSO 4 or KMnO 4 quickly diffuse in between the particles of water to form a solution.
- (ii) Diffusion of liquids into liquids: When water is added to alcohol or vice-versa, the two liquids quickly diffuse into each other to form a solution.
- (iii) Diffusion of gases into liquids: Some gases especially O 2 and CO 2 diffuse into water i.e. dissolve in water. So, that aquatic animal can breathe under water due to presence of dissolved oxygen in water.
Thus, solids, liquids & gases – all can diffuse into liquids. However, the rate of diffusion of liquids is much higher than that of solids.
(a) Rate of diffusion of different liquids :- Different liquids have different rates of diffusion. For example a drop of blue or red ink diffuses faster than a drop of honey into water.
(b) Rate of diffusion increase with rise in temperature :- Rate of diffusion increases with rise in temperature, hence sugar dissolves much more quickly in hot water than in cold water.
(c) Gases:
A gas is a state of matter, which has definite mass, but no definite shape and no definite volume.
e.g. : O 2 , N 2 , H 2 etc.
The force of attraction between gases molecules is very less
Properties of Gases:
(a) Gases neither have a definite shape nor a definite volume : Gases do not have a definite shape, but they acquire the shape of the vessel in which they are placed.
Similarly, gases do not have a definite volume, but attain the volume of container to which they are transferred.
(b) Gases have maximum fluidity and least rigidity : The gases have high fluidity (property to flow) and least rigidity (tendency to maintain shape) due to large inter particle space and weak inter particle forces of attraction in them.
(c) Gases are highly compressible : The gases are highly compressible due to large inter particle spaces in them. Due to high compressibility, large volume of a gas can be compressed into a small cylinder and transported easily. e.g. L.P.G. gas & O 2 supplied to hospitals in cylinders is compressed gas. Similarly these days, compressed natural gas (CNG) is used as a fuel in vehicles.
|
|
Gases are highly compressible while liquids are almost incompressible, while solids are completely incompressible. Due to this property gases can be kept in a small container at high pressure. |
This can be explained by the following experiment.
Experiment to study the compressibility of solids, liquids & gases : Take three syringes (about 100ml) and close their nozzles by rubber corks. Now remove the pistons from all syringes. Fill some water (liquid) in second syringe and chalk pieces (solid) in the third & leaving first syringe untouched. Now insert pistons back into syringes.
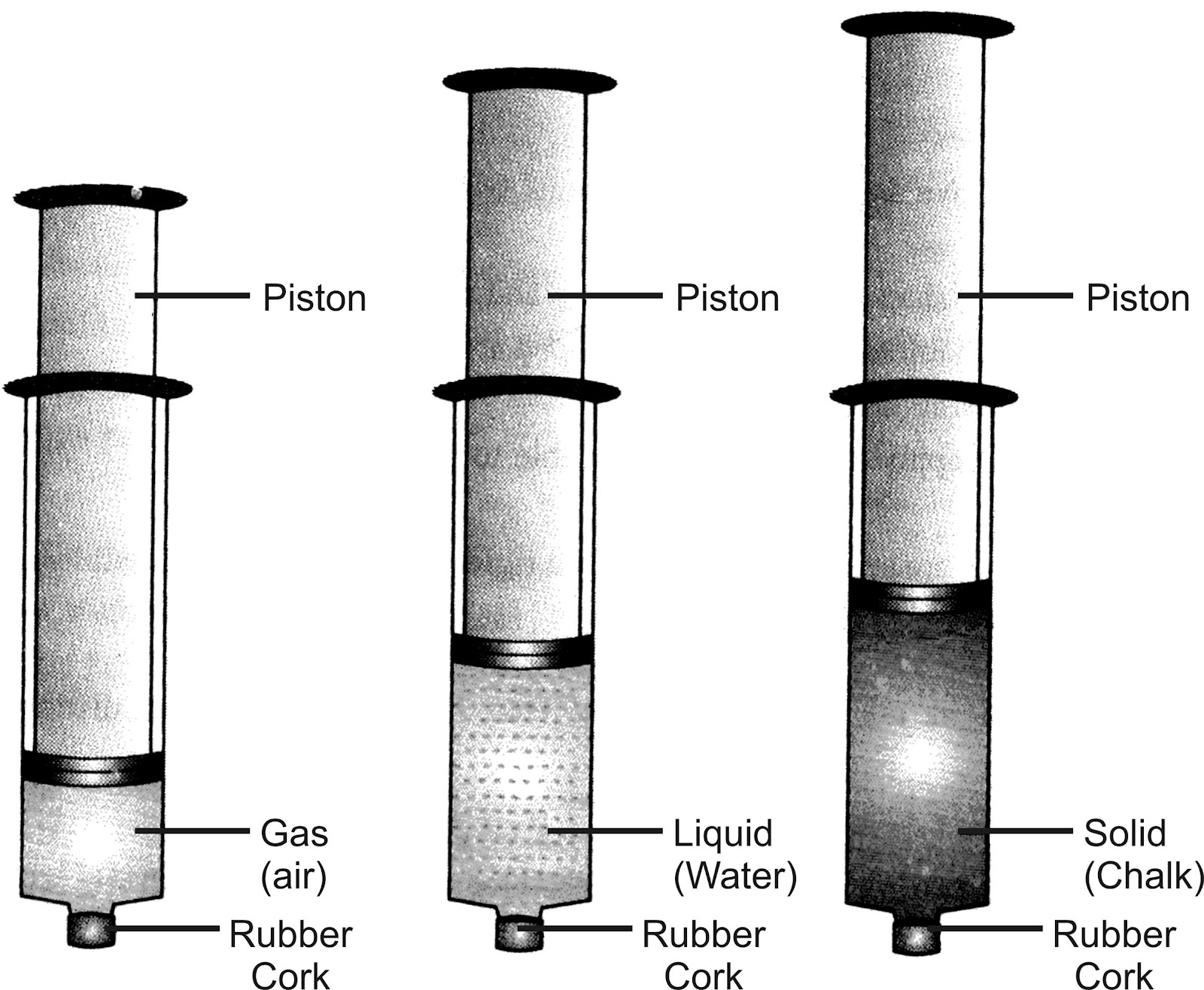
Observation and explanation: The piston of first syringe (left untouched) which contained air (gas) was easily pushed in. The piston of the second syringe which contained water (liquid) was pushed in only a little, while the piston of the third syringe which contained chalk pieces could not be pushed in at all. Thus, air is easily compressible, water is almost incompressible, while chalk pieces are completely incompressible.
Conclusion: The spaces between particles of gases are maximum, intermediate in liquids and minimum in solids. Thus, gases are highly compressible, liquids are almost incompressible, while solids are completely incompressible.
(d) Gases have low density : Gases have low density as compared to solids and liquids due to large inter molecular spaces in them. i.e. mass per unit volume of a gas is small and hence gases have low density.
(e) The Kinetic energy of particles in the gaseous state is quite high :- Due to large inter particle distances and weak forces of attraction, the particles of a gas can move freely & thus, have large rotational, translational and vibrational motion and due to large translational motion, their kinetic energy is quite high which can be further increased by increasing the temperature of gas.
(f) Gases exert pressure : Due to larger inter particle distances and weaker inter particle forces of attractions, particles of a gas are moving continuously in different directions with different velocities. Due to this random motion, the particles of gas collide with each other and also with the walls of the containing vessel. Due to these collisions, the particles of the gas exert a force on the walls of the container. This force per unit area exerted by the particles of the gas on the walls of containing vessel is called the pressure of the gas.
|
|
Random motion means motion in different directions with different velocities. The random motion of particles of a gas is due to larger inter particle distances and weaker inter particle forces of attraction between them, unlike liquids & solids. |
The motion and inter particle distances in solids, liquids & gases are as shown in fig.
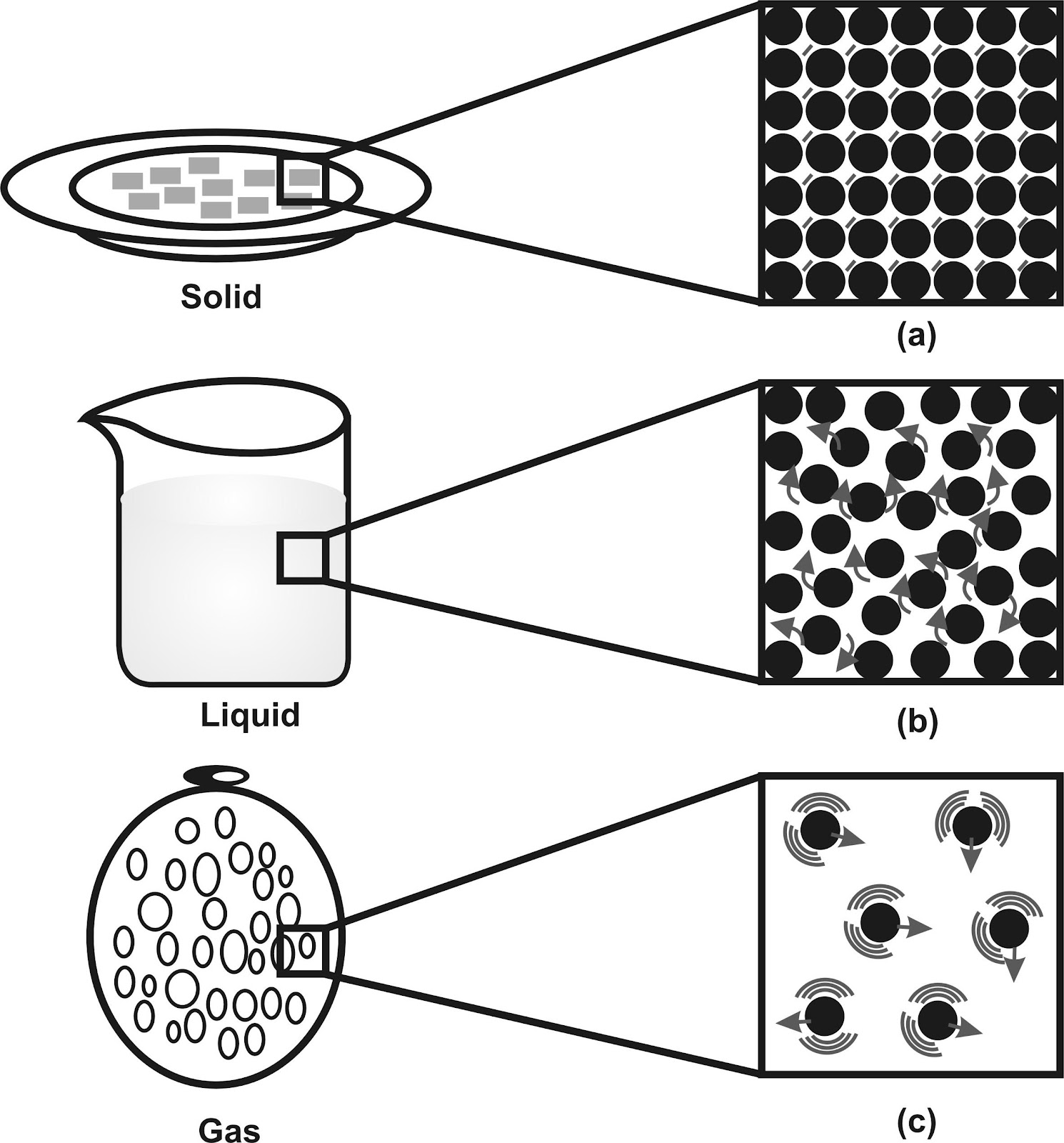
a, b and c show the magnified schematic pictures of the three states of matter.
The motion of the particles can be seen and compared in the three states of matter.
(g) Gases diffuse very rapidly : Due to random motion, the particles of one gas readily move into spaces between the particles of the other gas. (called diffusion)
Thus, gases diffuse very rapidly, rate of diffusion increases with increase in temperature.
The most familiar example of diffusion of gases is found in our homes, e.g. we come to know what is cooked in the kitchen without even entering there, by the smell due to rapid diffusion. (i.e. rapid intermixing of particles of aroma with particles of air). Since rate of diffusion becomes faster at high temperature the smell of hot cooked food travels faster than that of the cold food.
|
|
The rate of diffusion of a gas is inversely proportional to the square root of its density, this is called Grahm’s law of diffusion.
r α
where r= rate of diffusion P = pressure M = Molecular weight of gas |
Comparison of the characteristics of three states of matter.
|
S.No. |
Property |
Solid state |
Liquid state |
Gaseous state |
|
1 |
Interparticle spaces |
Very small spaces |
Comparatively large spaces |
Very large spaces |
|
2 |
Interparticles forces |
Very strong |
Weak |
Very weak |
|
3 |
Nature |
Very hard and rigid |
Fluid |
Highly fluid |
|
4 |
Compressibility |
Negligible |
Very small |
Highy compressible. |
|
5 |
Shape and volume |
Definite shape and volume |
Indenfinite shape, but definite volume |
Indefinite shape as well as volume |
|
6 |
Density |
High |
Less than the density in solid state |
Very low density |
|
7 |
Kinetic energy |
Low |
Comparatively high |
Very high |
|
8 |
Diffusion |
Negligible |
Slow |
Very fast |
|
|
Bose – Einstein-condensate (BEC): It is the fifth state of matter obtained by cooling extremely low density gas to super low temperature. |