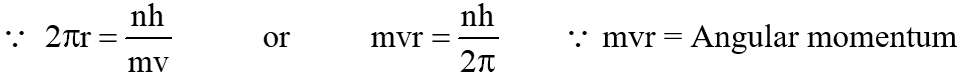Atom De-Broglie Concept : To understand the quantum mechanical model of an atom, let's start with the atom's basic structure. Imagine the atom as a tiny solar system, with a nucleus at the centre composed of protons and neutrons, surrounded by a cloud of electrons orbiting in regions known as orbitals. Unlike the classical model of the atom, which depicts electrons orbiting the nucleus in fixed paths like planets around the sun, the quantum mechanical model describes electrons as existing in probability clouds, known as orbitals. These orbitals represent regions in space where electrons are likely to be found rather than definite paths.
Atom De-Broglie Concept
Atom De-Broglie Concept model consists of the following
(A) de-Broglie concept (Dual nature of Matter)
(B) Heisenberg's Uncertainty principle.
Atom De-Broglie Concept (Dual nature of Matter)
Atom De-Broglie Concept (Dual nature of Matter) : One of the fundamental principles of the quantum mechanical model is the wave-particle duality of electrons. This concept suggests that electrons exhibit both wave-like and particle-like behaviour, depending on how they are observed. Electrons can behave as waves, spreading out over space, or as particles, localized at specific points.
1. The wave nature of light rays and X-rays is proved on the basis of their interference and diffraction and many facts related to radiations can only be explained when the beam of light rays is regarded as composed of energy particles or photons whose velocity is 3 × 10 10 cm/s.
2. According to de-Broglie, the wavelength λ, of an electron is inversely proportional to its momentum p.
(Here h = Planck's constant, p = momentum of electron)
-
Momentum (p) = Mass (m) × Velocity (v)
3. The above relation can be proved as follows by using Einstein's equation, Planck's quantum theory and wave theory of light.
Einstein's equation, E = mc 2 where E is energy, m is mass of a body and c is its velocity.
-
E =
= h ×
(According to Planck's quantum theory) …(i)
and
(According to wave theory of light)
But according to Einstein's equation, E = mc 2 …(ii)
From equation (i) & (ii): mc
2
= h ×
or mc =
or
4. It is clear from the above equation that the value of λ decreases on increasing either m or v or both. The wavelength of many fast-moving objects like an aeroplane or a cricket ball, is very low because of their high mass.
Bohr's Theory And Atom De-Broglie Concept Relationship
1. According to de-Broglie, the nature of an electron moving around the nucleus is like a wave that flows in circular orbits around the nucleus.
2. If an electron is regarded as a wave, the quantum condition as given by Bohr in his theory is readily fulfilled.
3. If the radius of a circular orbit is r, then its circumference will be 2πr.
4.
We know that according to Bohr theory, mvr =
or
(
∵
mv = p; momentum)
or
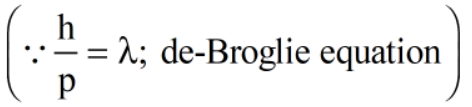
2πr = nλ (where n = total number of waves 1, 2, 3, 4, 5, …∞ and λ = Wavelength
Thus mvr = Angular momentum, which is a integral multiple of
6. It is clear from the above description that according to de-Broglie there is similarity between wave theory and Bohr theory.
Important Points For Atom De-Broglie Concept
Atom de-Broglie wavelength in terms of kinetic energy:
Kinetic Energy (K.E.) =
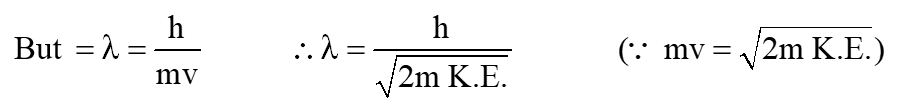 a charged particle carrying Q coulomb charge is accelerated by applying potential difference of V volts, then:
a charged particle carrying Q coulomb charge is accelerated by applying potential difference of V volts, then:
K.E. = Q × V Joule
But
The wave nature of electron was verified experimentally by Davisson and Germer.
Atom de-Broglie hypothesis is applicable to macroscopic as well as microscopic objects but it has no physical significance for macroscopic objects.
Atom De-Broglie Concept Solved Examples
Q. 1 : What will be the wavelength of a ball of mass 0.1 kg moving with a velocity of 10 m s –1 ?
Sol. According to de Broglie equation
Q.2 : Two particles X and Y are in motion. If the wavelength associated with particle X is 4 × 10 –8 m, calculate the wavelength associated with particle Y if its momentum is half of X.
Sol.
According to de Broglie equation
But
B = 2 A = 2 × 4 × 10 –8 m = 8 × 10 –8 m
Q.3 : Which of the following should be the wavelength of an electron if its mass is 9.1 × 10 –31 kg and its velocity is 1/10 of that of light and the value of h is 6.6252 × 10 –34 joule second?
(1) 2.446 × 10 –7 metre (2) 2.246 × 10 –9 metre (3) 2.246 × 10 –11 metre (4) 2.246 × 10 –13 metre
Sol.
Given that
m = 9.1 × 10
–31
kg,
of velocity of light
Or
meter second
–1
i.e., 3 × 10
7
metre second
–1
h = 6.6252 × 10 –34 joule second
= 0.2426 × 10 –10 metre = 2.426 × 10 –11 metre
Q. 4 : In Li 2+ ion electron jumps from 2 nd to 1 st orbit. If the emitted radiation is absorbed by H atom.
Calculate the de-Broglie wavelength of the ejected electron.
Sol.
Excess energy = 91.8 – 13.6 = 78.2 eV
Atom De-Broglie Concept FAQs
Q.1 : What is the De-Broglie Concept in relation to the quantum mechanical model of an atom?
Q.2 : What is the significance of the De-Broglie Concept in understanding atomic structure?
Q.3 : How does the De-Broglie Concept influence our understanding of electron orbits in atoms?
Q.4 : What experimental evidence supports the De-Broglie Concept?
Q.5 : Can the De-Broglie Concept be applied to other particles besides electrons?
Q.6 : What are some practical applications of the De-Broglie Concept?