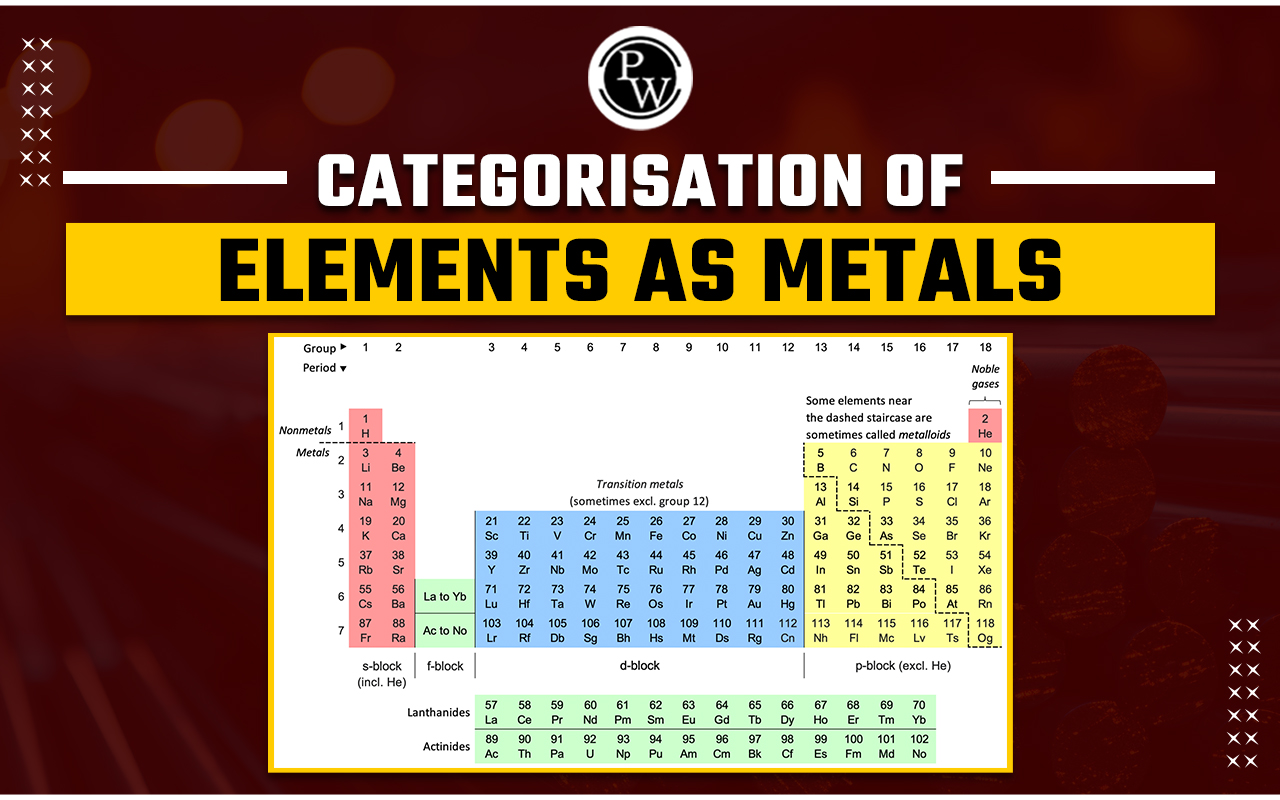
Categorisation Of Elements As Metals : The periodic table, a masterpiece in the realm of chemistry, serves as a systematic arrangement of elements based on their atomic structure and properties. One of the fundamental classifications that emerges from this arrangement is the categorisation of elements into metals, non-metals, and metalloids.
Metal : A metal is defined as an element that forms positive ions (cations) and has metallic bonds. They tend to lose electrons easily. Metals constitute a significant portion of the periodic table and are known for their distinctive properties. These elements typically have a shiny appearance, conduct electricity and heat efficiently, and exhibit high malleability and ductility.
Examples of metals include familiar elements like iron (Fe), copper (Cu), and gold (Au). The majority of elements on the left side of the periodic table fall into this category. The typical trend among metals is to exhibit high melting points; however, gallium and cesium stand out as exceptions with remarkably low melting points.
Further deviating from the general characteristics of metals, lithium, sodium, and potassium, it shows exceptional softness, allowing them to be easily cut with a knife. Additionally, alkali metals have low densities and melting points.
In contrast, certain metals like lead, mercury, titanium, and chromium defy the common expectation of high thermal conductivity, as they are regarded as poor conductors of heat. Notably, bismuth holds the distinction of being the poorest conductor of heat among the elements.
Non-Metals : Non-metals are elements that form negative ions(anions) by accepting or gaining electrons. They generally have 4, 5, 6, or 7 electrons in their outermost shell, so they tend to gain electrons during chemical reactions. In contrast to metals, non-metals encompass a diverse range of elements with unique properties.
Non-metals are generally poor conductors of electricity and heat, lack the characteristic metallic luster, and often appear in various states of matter at room temperature. Elements such as oxygen (O), nitrogen (N), and sulfur (S) are prime examples of non-metals. The majority of non-metals are situated on the right side of the periodic table.
In general, nonmetals tend to exhibit lower melting and boiling points. An exemplary nonmetal with distinctive properties is carbon, capable of existing in various allotropes. Diamond, one such allotrope, is renowned as the hardest natural substance, boasting exceptionally high melting and boiling points—deviating from the typical behavior of nonmetals.
Another allotrope of carbon, graphite, presents an additional exception as it serves as a proficient conductor of electricity. This further underscores the varied characteristics within the realm of nonmetals. Non-metals are less dense as compared to metals.
Metalloids : Metalloids are elements forming a simple substance having properties intermediate between those of a typical metal and a typical nonmetal. Metalloids, sometimes referred to as semi-metals, occupy a crucial intermediate position between metals and non-metals.
Elements classified as metalloids include silicon (Si), germanium (Ge), and arsenic (As). These elements display a combination of both metallic and non-metallic properties. Metalloids often serve as essential components in semiconductor materials, playing a vital role in electronic devices.
The classification of elements into metals, non-metals, and metalloids is based on various intrinsic characteristics. The location of an element in the periodic table, its electronic configuration, and its physical and chemical properties play pivotal roles in determining its classification.
The classification of elements has profound implications in our daily lives. Metals find application in construction, transportation, and the manufacturing of electrical appliances. Non-metals are essential in the formation of organic compounds, crucial for life processes, and are utilized in various industries. Metalloids, with their intermediate properties, contribute significantly to advancements in technology, particularly in the semiconductor industry.
