
Sodium Sulfide Formula: Sodium sulfide, also known as Na 2 S, is an inorganic chemical compound represented by the formula Na 2 S, which has gained significant recognition within the organic chemical industry. It serves as a potent alkaline solution, emitting a foul odor resembling rotten eggs when exposed to moist air. Notably, in its solid-state, sodium sulfide appears yellow, while its solution is typically colorless. It is commonly referred to as "sodium sulfide flakes" in various grades.
Also Check – Internal Energy Formula
What Is Sodium Sulfide?
Sodium sulfide holds importance in the realm of organic chemistry. Its chemical formula is Na 2 S, and it can also exist as its hydrate, Na 2 S 9 H 2 O. Both anhydrous and hydrous forms of this compound manifest as colorless solids. Sodium sulfide is a water-soluble alkaline substance, producing a highly basic solution. When Na 2 S and its hydrates come into contact with moist air, they undergo a reaction that generates hydrogen sulfide, emitting an unpleasant odor reminiscent of rotten eggs. In solution, sodium sulfide takes on a solid yellow form and is commercially available in various grades, commonly referred to as sodium sulfide flakes. The IUPAC designation for sodium sulfide is Disodium sulfide. Sodium sulfide is characterized by an oxidation number of -2 and possesses a pH value of 10.4.Also Check – Ionization Energy Formula
Sodium Sulfide Structure
Sodium sulfide adopts the antifluorite structure, wherein the positions of anions and cations are interchanged. In this arrangement, sodium ions (Na + ) occupy the sites typically reserved for fluoride ions, while sulfide ions (S 2- ) take the places typically held by calcium ions (Ca 2+ ).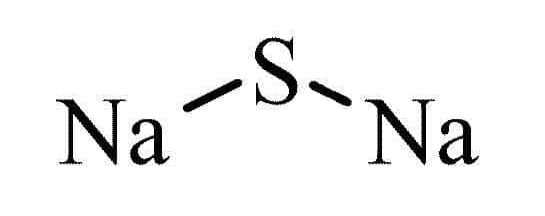
Physical Properties of Sodium Sulfide
Chemical Formula: Na 2 S
Density: 1.86 grams per cubic centimeter (gm/cm3)
Molar Mass: 78.0452 grams per mole (g/mol)
Autoignition Temperature: Above 480°C (896°F; 753 K)
Melting Point: 1,176°C (2,149°F)
Odor: Exhibits the scent of rotten eggs
Appearance: Appears as anhydrous yellow crystalline solids
Oxidation Number: -2
pH Value: 10.4
Solubility: Insoluble in ether; slightly soluble in alcohol
Also Check – Mass Percent Formula
Chemical Properties of Sodium Sulfide
In an aqueous medium, sodium sulfide dissolves and forms the necessary ions:
Na 2 S + H 2 O → 2Na+ + HS – + OH –
Upon heating, it undergoes rapid oxidation, resulting in the formation of sodium carbonate and sulfur dioxide.
2Na 2 S + 3O 2 + 2CO 2 → 2Na 2 CO 3 + 2SO 2
Interaction with sulfur leads to the generation of polysulfides:
2Na 2 S + S 8 → 2Na 2 S 5
When subjected to oxidation by hydrogen peroxide, sodium sulfide is transformed into sodium sulfate:
Na 2 S + 4 H 2 O 2 → 4 H 2 O + Na 2 SO 4
Also Check – Molecular Speed Formula
Uses of Sodium Sulfide
Paper and Pulp Industry: Sodium sulfide helps turn wood into paper by breaking down a tough substance called lignin.
Clean Water: It's used to clean water by getting rid of oxygen, which can cause problems in water systems.
Textile Industry: Sodium sulfide is like a magic wand for making fabrics white and pretty.
Photography: It helps make black and white photos look better by changing their colors.
Rubber Stuff: It's important for making rubber materials and dyes used in rubber.
Other Jobs: Sodium sulfide has many other jobs, like separating valuable stuff from rocks, making colorful clothes, getting oil out of the ground, and making soap to clean things.
So, sodium sulfide is like a helper in various industries, making things like paper, water, clothes, photos, rubber, and more.
Sodium sulfide, represented by the chemical formula Na 2 S, is a versatile inorganic compound with significant importance in various industries. Its notable characteristics, including its strong alkaline properties, foul odor in moist air, and distinctive yellow appearance in solid form, make it a valuable substance in organic chemistry.
Sodium sulfide's unique antifluorite structure, where cations and anions exchange positions, adds to its chemical intrigue. With a pH value of 10.4 and an oxidation number of -2, it demonstrates specific chemical properties that find practical applications.
Its roles in industries such as paper and pulp, water treatment, textiles, photography, rubber production, and beyond highlight its versatility and significance. Sodium sulfide is truly a valuable helper in numerous industrial processes.
Sodium Sulfide Formula FAQs
What is sodium sulfide?
What does sodium sulfide look like?
What is the pH value of sodium sulfide?
What is the chemical structure of sodium sulfide?
What are some common applications of sodium sulfide?










