
Zinc Acetate Formula: Zinc, denoted by the symbol Zn and having an atomic number of 30, is a chemical element. At room temperature, it exhibits slight brittleness and takes on a silver-grey appearance upon oxidation. This element belongs to the first group of Group 12 on the Periodic Table. Moving on to acetate, it is a compound represented by the formula C 2 H 3 O 2 - . Also known as the acetate ion or mono-acetic acid, it is a salt formed through the combination of acetic acid with various substances such as alkaline, metallic, soil texture, non-metallic, or other bases.
Zinc Acetate Formula
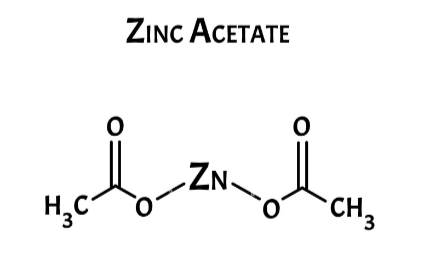
Zinc acetate, often referred to as zinc acetate (II), is a salt with the chemical formula Zn(CH 3 COO) 2 . It primarily consists of zinc bound to acetic acid. Typically, zinc acetate exists in its dihydrate form, Zn(CH 3 COO) 2 ·2H 2 O. Both the hydrated and anhydrous variations of zinc acetate find common use as dietary supplements. This comprehensive article aims to provide a detailed exploration of zinc acetate.
Structure of Zinc Acetate
Within zinc acetate, zinc coordinates with the four oxygen atoms from acetic anhydride, establishing a tetrahedral environment. These tetrahedral polyhedra are interconnected by acetate ligands, resulting in the formation of diverse polymer structures. In the case of zinc acetate dihydrate, zinc adopts an octahedral configuration, with both acetate groups exhibiting bidentate properties.
Physical Properties of Zinc Acetate
- Name: Zinc acetate
- Appearance: White solid
- Molecular Weight: 219.50 g/mol (dihydrate)
- Density: 1.735 g/cm3
- Melting Point: 237°C
- Boiling Point: Decomposes
- Molecular Shape: Tetrahedral
- Solubility in Water: 43 g/100 mL
Chemical Properties of Zinc Acetate
Reaction with Bases: Zinc acetate reacts with bases like sodium hydroxide to yield zinc hydroxide and sodium acetate.
Zn(CH 3 COO) 2 + 2NaOH → 2CH 3 COONa + Zn(OH) 2
Pyrolysis of Zinc Acetate: Under reduced pressure, pyrolysis of zinc acetate results in the production of acetic anhydride and basic zinc acetate.
2 Zn(CH 3 COO) 2 → Zn 4 O(CH 3 COO) 6 + (CH 3 CO) 2 O
Preparation of Zinc Acetate
Zinc Reacts with Acetate:
Zinc acetate is produced when zinc undergoes a reaction with acetate, accompanied by the liberation of hydrogen gas. The reaction can be represented as follows:
Zn + CH 3 COOH → Zn(CH 3 COO) 2 + H 2
Zinc Oxide and Acetic Acid Reaction:
Zinc acetate is formed when zinc oxide reacts with acetic acid, releasing water molecules in the process. The reaction proceeds as follows:
ZnO + 2CH 3 COOH → Zn(CH 3 COO) 2 + H 2 O
Uses of Zinc Acetate
Zinc acetate finds its primary and foremost application in the realm of dietary supplementation, where it serves as a valuable nutritional component. Beyond this pivotal role, zinc acetate assumes multiple functions in diverse domains:
Cold Treatment: It plays a significant role in assisting individuals in alleviating symptoms associated with common colds, contributing to their overall well-being during periods of illness.
Zinc Deficiency Resolution: In instances where individuals exhibit a deficiency in zinc levels within their bodies, zinc acetate is administered as a remedial measure to rectify and restore the adequate levels of this essential mineral.
Wilson's Disease Management: As a critical component of the comprehensive treatment strategy for Wilson's disease, zinc acetate takes on the task of curbing the body's absorption of copper. Typically, it is administered in the form of an oral supplement, thus actively participating in the management of this challenging medical condition.
Dermatological Applications: Within the cosmetic and medical sectors, zinc acetate assumes a pivotal role in topical treatments aimed at addressing acne-related issues. It frequently makes its appearance in various formulations such as lotions and ointments, and is even combined with antibiotics to enhance its therapeutic efficacy in combating acne.
Industrial Wood Preservation: In the industrial landscape, zinc acetate serves as an invaluable agent for wood preservation. Its use in this context helps safeguard wood materials from deterioration and decay, contributing to the longevity and durability of wooden structures and products.
zinc acetate is a versatile compound with a spectrum of applications, ranging from its fundamental role in dietary supplementation to its involvement in medical treatments, dermatological solutions, and industrial processes, all of which collectively underscore its significance across various domains.
Zinc Acetate Formula FAQs










