
ICSE Worksheet for chapter-9 Ammonia class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of ICSE Board Worksheet for Class 10 Chemistry . Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Standard 10 students can practice questions and answers which are given here for Chemistry in Grade 10 that will help them to improve their knowledge of all important chapters and their topics. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
If any students need to take the online test to check their concepts or undertstanding then they can visit Chemistry Quiz for Class 10 .
Summary
- Introduction
- Laboratory method-
- Ammonium salt with alkali
- Metal nitrides and water
- Manufacture of Ammonia by Haber’s process
- Properties, tests and uses of ammonia.
Section - 1
Q1. Give the name of a hydride of nitrogen.
- Which reactants are used in laboratory preparation of ammonia?
- What are the conditions required for the synthesis of ammonia in Haber’s process?
- Which compound is used in drying ammonia?
- What is the vapour density and nature of ammonia?
- Name two nitrides from which ammonia can be prepared?
Q2. Which feature of the ammonia molecules leads to the formation of the ammonium ion when ammonia dissolves in water?
- Name the other ion formed when ammonia dissolves in water.
- Give one test that can be used to detect the presence of the ion produced in (ii).
Q3. Gas B turns red litmus paper blue.
- What is the name of gas B?
- Write the equation for the reaction that takes place when gas B is passed over heated copper oxide.
Q4. What is the difference between chemical nature of an aqueous solutions of hydrogen chloride and an aqueous solutions of ammonia?
Section - 2
Q5. When an aqueous solution of ammonia is added to a solution of ferric chloride, what is observed?
Q6. Explain the following observations:
- On inverting a jar of ammonia in a trough of water, it is quickly filled with water.
- A fertilizer containing an ammonium salt gets spoiled, if accidently mixed with slaked lime.
- When a glass rod dipped in hydrochloric acid is introduced into a gas jar full of ammonia, dense white fumes are produced.
Q7. Complete the following equations.
- Mg 3 N 2 + 6H 2 O →
- 2NH 3 + 3CuO →
- 8NH 3 + 3Cl 2 →
- 4NH 3 + 5O 2 →
How would you obtain the compound magnesium nitride?
- What property of ammonia is illustrated by reaction (i) (b) above?
- What important industrial process starts with reaction (i) (d) above takes place? Name the catalyst used.
- During laboratory preparation how is ammonia dried and collected?
Q8. Distinguish between liquid ammonia and liquid ammonia fort is.
Q9. Write the equation for the following reaction : (ICSE 2009)
- Aluminium nitride and water
- Magnesium nitride and water
Q10. State your observation when ammonia gas is burnt in an atmosphere of oxygen in the absence of a catalyst.
Q11. The following reactions are carried out:
A: Nitrogen + Metal →Compound F
B: F + Water →Ammonia + E
C: Ammonia + Metal oxide →Metal + Water + Nitrogen
- One metal that can be used for the reaction is calcium:
- Name two other metals that can be used.
- Write the balanced equation when nitrogen and calcium react.
- Write the correctly balanced equation for reaction B where F is the compound formed between nitrogen and calcium.
- What property of ammonia demonstrated by reaction C?
- Ammonia burns in oxygen and the combustion, in the presence of a catalyst, may be represented by:
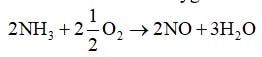
- What mass of steam is produced when 1.5 gm of nitric oxide is formed?
- What volumes of oxygen at STP, is required to form 10 moles of products?(H=1,N=14,O=16,1 mole of a gas occupies 22.4 litre at STP).
Q13.
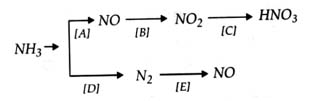
- Show by balanced equations how would you carry out the conversions A, B, C, D and E.
- How can ammonia be separated from unreacted nitrogen and hydrogen?
Q14. How would you obtain nitrogen from ammonia? Give equation.
Q15. Two gases combine together to form an important fertilizer. Write equation and name the fertilizer. State the temperature and pressure used for the reaction.
Q16. Ammonia is prepared on a large scale by synthesis.
- Name the process.
- What are the sources and preparations of nitrogen and hydrogen?
- What are the conditions for the combination?
- How is ammonia prepared from the salt ammoniac? Give an equation
- What happens when nitrogen dioxide is bubbled through water? Which property of the gas is exhibited? Give an equation for the reaction
Q17. A glass rod, moistened with conc. Hydrochloric acid, is held at the mouth of a gas filled with ammonia, what will you observe and why?
Ammonia can be oxidized to nitric oxide. Mention the reaction with equation and state the conditions under which it occurs.
A green solid W on heating gave off a gas X, which turned lime water milky, and a black solid Y. This black solid, Y dissolved in warm dilute sulphuric acid to form a blue solution Z. Give names for substance W to Z with the help of reactions.
- Solid W
- Gas X
- Solid Y
- Solution Z
Q18. Why distillation or boiling cannot be used to concentrate nitric acid beyond a certain concentration?
Give the following data in connection with the Ostwald’s Process of manufacturing nitric acid.
- The source of ammonia used.
- The catalyst used.
- The volume ratio of ammonia: air taken.
- The product of the oxidation of ammonia.
- Temperature maintained during the oxidation of ammonia.
- How is nitric oxide converted to nitric acid?
Why is the round bottom flask kept in an inclined position facing down while preparing
NH 3 gas by the reaction between slaked lime and ammonium chloride?
Q19. Why are drying agents like conc. H 2 SO 4 , fused CaCl 2 , P2O 5 not sutiable for drying ammonia? Give equations. Name a sutiable drying agent for ammonia.
To prepare an aqueous solution of ammonia, the dissolution of the gas in water is carried out using a funnel arrangement. State two reasons.
Show by balanced equations, the preparation of the following fertilizer:
- NH 4 NO 3
- CO(NH 2 ) 2
- CaCN 2









