Chemical Properties Of Acids
Acid Base And Salt of Class 10
REACTION OF ACIDS WITH METALS:
When acid reacts with a metal, then a salt and hydrogen gas are formed.
i.e. Metal + Acid → Salt + Hydrogen gas
⇒ Reaction of dil H 2 SO 4 with zinc metal.
Experiment: Take about 5 ml of dil H 2 SO 4 in a test tube and add a few pieces of zinc granules in it. Pass the gas evolved through soap solution. The soap bubbles filled with gas rise.
Test for gas: Bring a burning candle near the gas filled soap bubble.
Observation: The gas present in soap bubble burns with pop sound which shows the gas evolved during reaction is hydrogen gas.
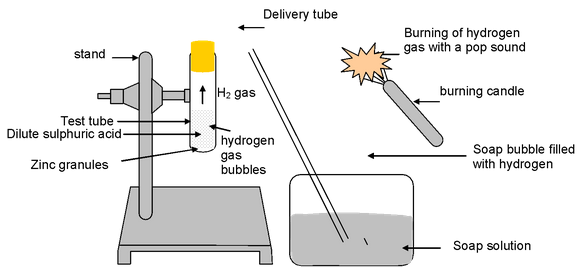
Figure-Reaction of Zn granules with dil. H 2 SO 4 and test for hydrogen gas by burning
The reaction involved is
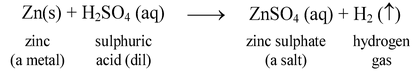
In this reaction more active metal zinc displaces less active hydrogen from H2SO4 and this hydrogen is evolved as gas.
Thus it is an example of displacement reaction.
Some more examples of reaction of different metals with a particular acid:
Ex 1. Mg(s) + 2HCl (aq) &arr; MgCl 2 (aq) + H 2 (g)
Magnesium hydrochloric acid magnesium chloride hydrogen
(a metal) (dil) (a salt) gas
Ex 2. Zn (s) + 2HCl(aq) → ZnCl 2 (aq) + H 2 (g)
zinc hydrochloric acid zinc chloride hydrogen
(a metal) (dil) (a salt) gas
Ex 3. Fe(s) + 2HCl(aq) → FeCl 2 (aq) + H 2 (g)
iron hydrochloric acid iron(II) chloride hydrogen
(a metal) (dil) (a salt) gas
Ex 4. Cu + 2HCl(aq) → no reaction
copper hydrochloric acid
(a metal) (dil)

|
The order of reactivities of above metals with same acid (dil HCl) is Mg > Zn > Fe > Cu i.e. these metals do not react with same acid with same vigour. |
It is observed that at room temperature
- Mg reacts most vigorously
- Zn reacts less vigorously than Mg
- Fe reacts slowly
- Cu does not react at all
Conclusion: From above reactions we reach to a conclusion that all metals do not react with same acid with same vigour.
The reason is the different reactivities or activities of metals towards acid.
In the above reactions we observe that metals like Mg, Fe, Zn being more active than hydrogen, displaces hydrogen from acid HCl and release H2 gas. Thus above reactions are displacement reactions, Cu being less reactive than hydrogen, cannot displace hydrogen from dil HCl. Thus no reaction takes place.
REACTION OF ACID WITH METAL CARBONATES AND METAL HYDROGEN CARBONATE (OR METALBICARBONATES):
When an acid reacts with a metal carbonate or metal hydrogen carbonate (metalbicarbonate), then a salt, CO 2 gas and H 2 O are formed.
|
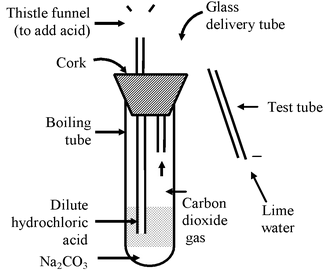
i.e. Metal carbonate + Acid → Metal Salt + CO 2 + H 2 O Metal hydrogen carbonate (or metal bicarbonate) + Acid → Metal Salt + CO 2 + H 2 O ⇒ Reaction of sodium carbonate (Na 2 CO 3 ) or sodium hydrogen carbonate (NaHCO 3 ) with dil HCl. Experiment: Take about 0.5g of Na 2 CO 3 or NaHCO 3 in a test tube and add about 2 ml of dil HCl acid to it. Pass the gas evolved through lime water (taken in another test tube). Observation: The lime water turns milky, showing that the gas evolved is CO 2 gas. |
Figure-Carbon dioxide gas (formed by the action of dil. HCl and Na 2 CO 3 ) being passed through lime water |
The reactions taking place are
Na 2 CO 3 (s) + 2HCl(aq) → 2NaCl(aq) + H 2 O(l) + CO 2 (g)
sodium
carbonate hydrochloric acid sodium chloride water carbondioxide
(dil) (a salt)
Ca(OH 2 ) (aq) + CO 2 (g) → CaCO 3 (s) + H 2 O(l)
lime water carbondioxide calcium carbonate water
(white ppt) (milky suspension)
|
|
(i) The lime water on passing CO 2 gas turns milky due to formation of white ppt. of CaCO 3 (insoluble in water) having milky appearance. (ii) On passing excess of CO 2 gas through lime water, milkiness disappears due to dissolution of white ppt of CaCO 3 and clear solution is formed due to formation of soluble calcium bicarbonate [Ca(HCO 3 ) 2 ] CaCO 3 (s) + H 2 O(l) + CO 2 (g) → Ca(HCO 3 ) 2 (aq) calcium carbonte water carbondioxide calcium bicarbonate (white ppt) (soluble in water) (insoluble in water) colourless |
Ex 1. MgCO 3 (s) + 2HCl (aq) → MgCl 2 (aq) + H 2 O (l) + CO 2
(dil)
Mg(HCO 3 ) 2 (aq) + 2HCl (aq) → MgCl 2 (aq) + 2H 2 O (l) + 2CO 2
(dil)
Ex 2. CaCO 3 (s) + H 2 SO4(aq) → CaSO 4 (aq) + H 2 O (l) + CO 2 (g)
(dil)
Ca(HCO 3 ) 2 (aq) + H2SO 4 (aq) → CaSO4(aq) + 2 H 2 O (l) + 2CO 2 (g)
(dil)
Ex 3. ZnCO 3 (s) + 2HCl (aq) → ZnCl2(aq) + H 2 O (l) + CO 2 (g)
(dil)
Zn(HCO 3 ) 2 (aq) + 2HCl(aq) → ZnCl 2 (aq) + 2H 2 O (l) + 2CO 2 (g)
(dil)
REACTION OF ACIDS WITH BASES (NEUTRALIZATION REACTION)
When an acid reacts with a base then a salt and water are formed, i.e
Acid + Base → Salt + water
This reaction is called neutralization reaction, because when acid and base react with each other, they neutralize each other’s effect (i.e base destroys the acidic property of acid and acid destroys the basic property of base).
⇒ Reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH).
Experiment: Take about 10 ml of dil NaOH solution in a conical flask and add 2-3 drops of phenolphthalein indicator to it. The solution will turn pink (showing that it is basic in nature). Now add dil HCl solution from burette into flask slowly till the pink colour in the solution disappears.
Observation: This point (at which pink colour disappear) is called end point.
At end point:
(i) The dil NaOH solution in flask has been completely neutralised by dil HCl solution added from burette, dil NaOH has completely reacted with dil HCl.
(ii) [H+] = [OH–] (in case of NaOH & HCl)
(from acid) (from base)
The chemical reaction can be written as
NaOH (aq) + HCl (aq) → NaCl(aq) + H 2 O(l)
sodium hydroxide hydrochloric acid sodium chloride water
(base) (acid) (salt)
This reaction of acid and base to form salt and water is called neutralization reaction or neutralization of base by an acid
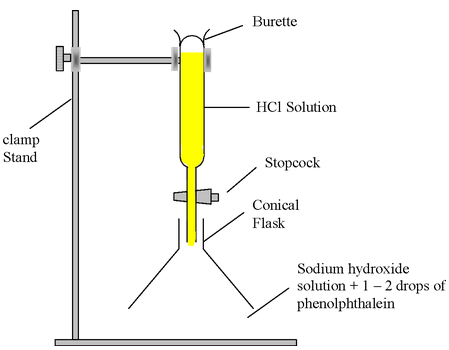
Figure-Neutralization of NaOH solution by HCl solution using phenolphthalein indicator
In the solution, NaOH, HCl and NaCl ionize completely into ions, so the above reaction can be written as:
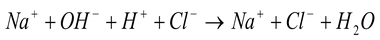
Canceling out the common ions on both sides, we get:
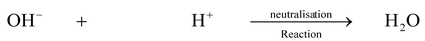
hydroxide ion hydrogen ion water
(from base) (from acid)
Hence, neutralization may also be defined as the reaction between H+ ions given by acid with the OH - ions given by base to form water.
REACTION OF ACIDS WITH METALLIC OXIDES:
Acid react with metal oxide to form salt and water.
i.e. Metal oxide + Acid → Salt + Water
This reaction is similar to the neutralization reaction between acid and a base to form salt and water. Thus, the reaction between acids and metal oxides is a kind of neutralization reaction and shows that metallic oxides are basic oxides.
⇒ Reaction of copper (II) oxide with dilute hydrochloric acid:
Experiment: Take about 1- 2g of copper (II) oxide (black in colour) in a beaker. Add dil HCl slowly with constant stirring.
Observation: Black CuO dissolves in dil HCl and a bluish green solution is formed due to formation of copper (II) chloride (CuCl 2 ) as salt.
The reaction taking place is:

copper (II) oxide hydrochloric acid copper (II)chloride water
(black) (salt)
|
|
In general Mineral Acids are Strong Acids while Organic Acids are Weak acids. |