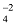Monoatomic Ions and Polyatomic Ions
Atom and Molecule of Class 9
Monoatomic ions : Those ions which are formed form single atoms are called monoatomic ions or simple ions.
e.g. Na + , Mg 2+ etc.
Polyatomic ion
Ions formed from a group of atoms carrying a charge (either negative or positive) are known as a polyatomic ion or compound ion.
For example
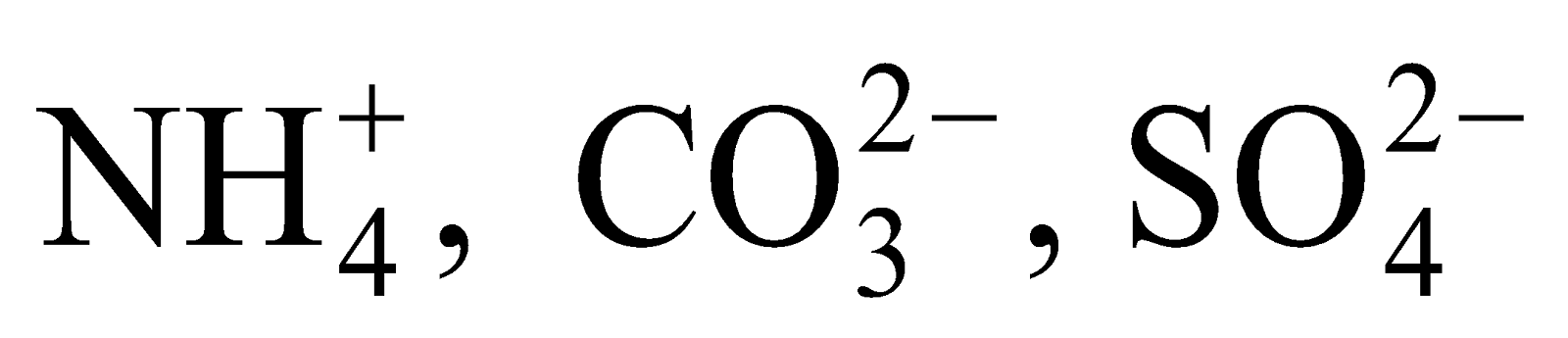 .
.
IONIC COMPOUNDS
Those compounds which are made up of ions (cations and anions) and are held together by strong electrostatic forces of attraction are called ionic compounds.
The forces which hold the ions together in an ionic compound are known as ionic bonds or electrovalent bonds.
e.g. Calcium nitrate Ca(NO 3 ) 2 is an ionic compound, whose one molecule is made up of one calcium ion (Ca 2+ ) and two nitrate ions (NO 3 ), making the overall charge on calcium nitrate zero.
Overall charge on an ionic compound is always zero
Some of the common ionic compound, their formula and the ions present in them are given below :
Some Ionic compounds
|
Name |
Formula |
Ions present |
|
1. sodium chloride |
NaCl |
Na + and Cl − |
|
2. Potassium chloride |
KCl |
K + and Cl − |
|
3. Ammonium chloride |
NH4Cl |
NH4 + and Cl − |
|
4. Magnesium |
MgCl2 |
Mg 2+ and Cl − |
|
5. Calcium chloride |
CaCl2 |
Ca 2+ and Cl − |
|
6. Magnesium oxide |
MgO |
Mg 2+ and O 2 − |
|
7. Calcium oxide |
CaO |
Ca 2+ and O 2 − |
|
8. Aluminium oxide |
Al2O3 |
Al 3+ and O 2 − |
|
9. Sodium hydroxide |
NaOH |
Na + and OH − |
|
10. Copper sulphate |
CuSO4 |
Cu
2+
and SO
|
|
11. Calcium nitrate |
Ca(NO2)2 |
Ca 2+ and NO 3 − |
As discussed earlier a compound is always made up of the same elements combined together in a fixed proportion by mass. For example Hydrogen chloride contains hydrogen and chlorine in a fixed ratio of 1 : 35.5 by mass. Similarly calcium oxide contains calcium and oxygen in the fixed ratio of 40 : 16 or 5 : 2 by mass.
Some ionic compounds |
||
|
Ionic Compound |
Constituting Elements |
Ratio by Mass |
|
Calcium oxide Magnesium Sulphide Sodium chloride |
Calcium and oxygen Magnesium and sulphur Sodium and chlorine |
5:2 3:4 23:35:5 |
Formula Unit of Ionic Compounds :
The simplest combination of ions that produces and electrically neutral unit, is called a formula unit of the ionic compound. Molecular formula of ionic compounds cannot be determined because they consist of large no. of ions. So, ionic compounds are represented by formula unit.
e.g. Sodium chloride is an ionic compound which consists of a large number of Na + and Cl - ions (but they should be equal in number). So, the actual formula of sodium chloride should be (Na + )n(Cl - )n or (Na + Cl - )n, where ‘n’ is a large number. Nacl is the simplest formula of sodium chloride and thus, the formula unit of sodium chloride is NaCl.
Also Check