
Introduction
Carbon and its compound of Class 8
About Carbon and its compound
Carbon is the seventeenth most abundant element found in earth’s crust. It is present in the bodies of plants, animals all living organisms, and also in several non-living things. It is present in air as CO 2 . It forms the largest number of compounds. Compounds of carbon are studied as a separate branch of chemistry known as Organic Chemistry. It exists in free as well as in combined form.
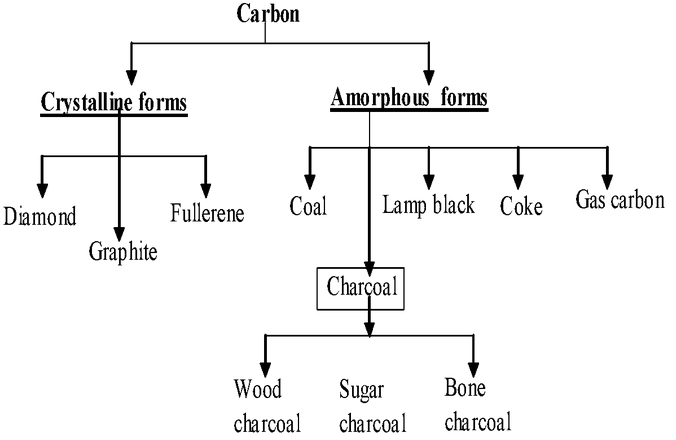
Diamond, graphite and coal are the free forms of carbon. Marble, paper, cloth and sugar are the combined forms or compounds of carbon. Carbon has a unique property that it can join with other carbon atoms to form carbon - carbon bonds.This can result in the formation of compounds with long chains. This property is called catenation.
The most important combination of carbon is with hydrogen to form a set of compounds known as Hydrocarbons .To score More in your class 8 prefer NCERT solutions for class 8 and go through with chemistry notes prepared by Physics Wallah.
OCCURRENCE OF CARBON
Carbon is widely distributed in nature, both in Free State and in the combined state.In free state it occurs as diamond, graphite and coal.
In the combined state, it occurs:
(A) In the solid state as:
(i) Carbonates in many minerals such as:
Limestone CaCO 3
Magnesite MgCO 3
Siderite
FeCO 3 also known as spathic iron.
Dolomite MgCO 3 .CaCO 3
Calamine ZnCO 3
(ii) In wood
(B) In the gaseous state as:
Carbon dioxide in small (0.04%) quantities in air.
Natural gas and petroleum gas.
All living systems are made up of carbon compounds such as fats, carbohydrates, proteins, vitamins, hormones etc.,
Similarly, fossil fuels such as petroleum, lignite, coal, etc., obtained from living matter are all carbon compounds.
In a nutshell, life as we live today would have been impossible without carbon compounds.
Carbon exist in different form, the following chat explain the different form of carbon
Frequently Asked Question (FAQs)
Q1. What are carbon and its compound?
Ans. Carbon compounds are defined as chemical compounds that contain carbon. There are more carbon compounds than any other chemical element other than hydrogen. Organic carbon compounds are much higher than carbon dioxide mixtures. Typically, carbon bonds and other elements are covalent bonds.
Q2. What is the use of carbon Class 8?
Ans. The use of carbon in class 8 are
- Carbon in coal, charcoal, and coke is a heating fuel.
- Carbon in graphite is used to make electrodes and writing pencils.
Q3. What are the four-carbon compounds?
Ans. The four major organic compounds in all living things are carbohydrates, lipids, proteins, and nucleic acid.
Q4. How do you read carbon and its compounds?
Ans. Carbon compounds are those whose molecules contain a carbon atom. They are the chemical substances where a carbon atom has bonded to an atom of another element. These compounds are generally organic. However, many are under the false impression can if a molecule contains carbon, it is organic.
Q5. What are the types of carbon compounds?
Ans. Carbon compounds may be organic, organometallic, or inorganic.
- Organic compounds: Organic compounds always contain carbon and hydrogen.
- Organometallic compounds: Organometallic compounds contain at least one carbon-metal bond.
- Inorganic carbon compounds : Inorganic compounds contain carbon but not hydrogen.
Q6. What are the properties of carbon compounds?
Ans. The properties of carbon compounds (covalent compounds) are as follows:
- They have both low melting points and boiling points.
- They possess a weak force of attraction between the molecules.
- They are non-conductors of electricity.
- They exist in solid, liquid, or gaseous states.
Also Check









