
Ionization Potential Or Ionization Energy
Periodic Properties of Class 11
The amount of energy required to remove the most loosely bound electron of the outermost shell (i.e. the outermost electron) from one mole of an isolated gaseous atom of an element in its ground state to produce a cation is known as ionization energy of that element. Ionization energy is generally expressed in electron volts, so it is also known as ionization potential. Energy required for the removal of first, second and the third electron from the gaseous atom is called first, second and third ionization energy respectively.
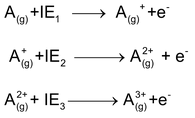
The order of first, second and third ionization energies may be given as, IE 1 < IE 2 < IE 3
This is because second and third electron is removed from unipositive and di-positive cations respectively. Effective nuclear charge increases with the increase of positive charge. So the attraction between the nucleus and the outermost electron increases and more energy is required for the removal of electron.
Factors Affecting Ionization Potential:
- Atomic radius: The values of ionization potential of an element decreases as its atomic radius increases. This is because the electrostatic force of attraction between the nucleus and the outermost electron decreases as the distance between them increases. So the energy required for the removal of electron will comparatively be less

Atomic radii increases
Li Na K Rb Cs → 520 496 419 403 374
ionizaton potential decrases
- Effective nuclear charge: The greater the effective charge on the nucleus of an atom, the more difficult it would be to remove an electron from the atom because electrostatic forces of attraction between the nucleus and the outermost electron increases. So the greater energy will be required to remove the electron.
Ionization potential α Effective nuclear charge (Zeff)
- Penetration effect of the orbitals: The order of energy required to remove electron from s,p,d-andƒ-orbitals of a shell is s>p>d>ƒ because the distance of the electron from the nucleus increases. For example – The value of ionization potential of Be(Z=4, 1s 2 2s 2 ) and Mg(Z=12, 1s 2 2s 2 2p 6 3s 2 ) are more than the I.P.s of B (Z=5, 1s 2 2s 2 2px1) and Al (Z= 13, 1s 2 2s 2 2p 6 3s 2 3px1) because the penetration power of 2s and 3s electrons is more than 2p and 3p electrons respectively. More energy will be required to separate the electrons from 2s and 3s orbitals.
- Shielding or screening effect: The shielding or screening effect increases if the number of electrons in the inner shells between the nucleus and the outermost electrons increases. This results in decreases of force of attraction between the nucleus and the outermost electron and lesser energy is required to separate the electron. Thus the value of I.P. decreases.
Ionization potential α 1/ Shielding effect
- Stability of half-filled and fully-filled orbitals: According to Hund's rule the stability of half filled and completely filled degenerate orbitals is comparatively high. So comparatively more energy is required to separate the electron from the atoms having half filled and full filled electronic configuration.
For example:
-
Removal of electron is comparatively difficult from the half filled configuration of N (Z=7, Is 2 2s 2 2px12py1pz1).
- The ionization potential of inert gases is very high due to stable ns2np6 electronic configurations.
PERIODICITY IN IONIZATION POTENTIAL
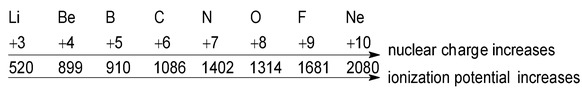
- For normal elements: One moving from left to right in a period, value of ionization potential of elements increases because effective nuclear charge also increases.
Exceptions:
- In a period, the ionization energy of IIA group elements is more than the elements of IIIA because penetration power of s-electrons is more than that of p- electrons. The value of ionization energy of Be(1s 2 2s 2 ) is more than B (1s 2 2s 2 px1) because the penetration power of 2s-electrons of Be is more than the 2px electrons of B. Moreover the electrons are paired i.e. completely filled s-subshell.
-
In a period, the ionization energy of VA elements is more than the elements of VIA because the half filled p
3
configuration of VA elements is comparatively of higher stability. VIA group elements (p4) have the tendency to acquire comparatively more stable (p3) configuration by the loss of one electron? Ionization energy of N(1s
2
2s
2
2px1py1pz1)>O
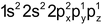
Thus P>S, As>Se.
But the value of I.P. of Sb (VA) & Te (VIA) and Bi (VA) & Po(VIA) are according to general rule i.e.
Sb (VA) <Te (VIA)
Bi (VA) < Po (VIA)
On moving from top to bottom in a group the value of I.P decreases because the atomic size increases.
Exceptions:
- In group IIIA the ionization potential of Al (13) is equal to the ionization potential of Ga(31). Before Ga (31) the electrons are filled in 3d – orbitals of ten transition elements. These 3d orbital electrons do not completely shield the 4p – electron. So the increase of +18 units in nuclear charge results in the greater increase of effective nuclear charge. Due to increase in nuclear charge the I.P. increases which counter balance the decrease in I.P. due to the increase in number of shells.
- The values of I.P. of Ti (81) and Pb (82) of sixth period is more than that I.P. values of In (49) and Sn (50) of same groups in period fifth. This is because of the electrons are filled in 4ƒ-orbitals before Ti (81) and Pb (82) which do not completely shield the outer electrons. Thus increase in + 32 units in nuclear charge results in the increase of ionization potential values
For transition elements: On moving from left to right in a transition series –
- As the atomic number increases the effective nuclear charge also increases. Hence the I.P. increases.
- The shielding effect of (n – 1)d electrons over ns electrons increases with the addition of electrons in (n-1)d orbitals. Hence the I.P. decreases.
- The increased values of I.P due to the increase of effective nuclear charge almost balances the decreased value of I.P. due to increase in shielding effect. There is a very small increase in the values of I.P. or it may be said that I.P. almost remains the same.
- In first transition series from Sc to Cr the value of I.P. increases because effect of increase in effective nuclear charge is more than the shielding effect. I.P. values of Fe, Co, Ni and Cu are almost same. Due to (n-1)d 10 ns 2 configuration of Zn, the first I.P. increases.
On moving from top to bottom in a group in transition series:
- In a group on moving from first to second transition series, the values of I.P decreases because atomic size increases.
- When moving from second to third transition series the value of I.P some what increases except IIIB group [Y(39) → La(57)]. This is because of 14 electrons are filled in 4ƒ-orbitals of lanthanides which do not shield the 5d electrons effectively. Thus the increase in +32 units in nuclear charge results in the increase of I.P., on moving from left to right this effect decreases and becomes negligible in the later part.
Applications of Ionization Potential:
- Metallic or electropositive character of elements increases as the value of ionization potential decreases. So in a group moving from top to bottom metallic or electropositive character increases because I.P. value decreases. In a period moving from left to right the values of I.P. increases so metallic or electropositive character decreases. Non metallic character increases.
- The relative reactivity of the metals increases with the decrease in I.P. values. The I.P. values of IA and IIA metals are comparatively low. So they are comparatively more reactive. The I.P. values of inert gases are very high. So they are almost inactive.
In a group moving from top to bottom the reactivity of metal atoms increases because their I.P. value decreases.
- The reducing power of elements increases as the values of I.P. decreases because tendency to lose the electron increases. The reducing power increases going down a group because the I.P. value decreases. Li is exception in IA group. The reducing power of Li is highest in its own group. The order of reducing Power of IA elements is as under
Li>Cs>Rb>K>Na
- Determination of oxidation state or valency electrons of an element –
(a) If the difference of two consecutive I.P.'s of an element is 16 eV or more, the lower oxidation state is stable. For e.g. the difference of first and second I.P. of Na is
42.4 eV which is more than 16 eV. So Na+ will be stable. It can also be explained from its electronic configuration
Na (11) = 1s 2 2s 2 2p 6 3s 1
Na+ = 1s 2 2s 2 2p 6
Neutral Na atom has the tendency to acquire the stable 2s 2 2p 6 configuration by the loss of one electron. Due to 2s 2 2p 6 configuration of Na+, the further separation of electron is difficult. So IA group metals form unipositive ions.
(b) If the difference of two consecutive I.P.s. of an element is 11.0 eV or less, the higher oxidation state is stable. For e.g. the difference of first and second I.P. of Mg is 7.4 eV which is less than 11.0 eV. So Mg2+ will be stable. It can also be explained on the basis of its electronic configuration.
The electronic configuration of Mg2+ is stable 2s 2 2p 6 configuration (Ne gas configuration)
Mg 2+ = 1s 2 2s 2 2p 6
So IIA group elements form di-positive ions.
(c) The difference of first and third I.P. of Al is 12.8 eV which is more than 11eV. Therefore first oxidation state of Al i.e. Al+ must be stable. In gaseous state Al+ is stable. This is due to the proportionate distribution of lattice energy and the difference of second and third I.P.s 9.6eV<11 eV.









