
NCERT Solutions for Class 10 Science Chapter 3: NCERT Solutions for Class 10 Science Chapter 3 "Metals and Non-metals," focuses on understanding the physical and chemical properties of metals and non-metals. Metals are generally malleable, ductile, lustrous, and good conductors of heat and electricity, while non-metals are brittle, non-lustrous, and poor conductors.
NCERT Solutions for Class 10 Science Chapter 3 explores the reactivity of metals, showing their reactions with oxygen, water, and acids. It also introduces the activity series of metals, explaining why some metals are more reactive than others. Non-metals are discussed in terms of their ability to gain electrons and form covalent bonds.Class 10 Science Chapter 3 Metals and Non-Metals
NCERT Solutions for Class 10 Science Chapter 3, "Metals and Non-metals," deals with the properties, reactions, and uses of metals and non-metals. Metals are generally solid, lustrous, ductile, malleable, and good conductors of heat and electricity, while non-metals are often gases or brittle solids, non-lustrous, and poor conductors.NCERT Solutions for Class 10 Science Chapter 3 explores the physical and chemical properties of both categories. Metals tend to lose electrons to form positive ions, and non-metals gain electrons to form negative ions, leading to the formation of ionic bonds. Important reactions such as reactions of metals with oxygen, water, and acids are highlighted. The activity series of metals is introduced, showing how reactivity influences their reactions.
NCERT Solutions for Class 10 Science Chapter 3 PDF
Understanding these concepts helps students grasp the real-world applications of metals and non-metals in industries and everyday life. Hence, here we have provided the NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals pdf for the ease of the students.NCERT Solutions for Class 10 Science Chapter 3 PDF
Class 10 Science Chapter 3 Question Answer
Below is the NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals -In-text questions set 1 Page number 40
1. Give an example of a metal which
(i) Is a liquid at room temperature?
(ii) Can be easily cut with a knife?
(iii) Is the best conductor of heat?
(iv) Is a poor conductor of heat?
Solution:
(i) Mercury is the metal which is liquid at room temperature (ii) Sodium and potassium are the metals which can be cut with a knife (iii) Silver is the best conductor of heat (iv) Mercury and lead are poor conductor of heat.2. Explain the meanings of malleable and ductile.
Solution:
- Metals which can be beaten to sheets are said to be malleable
- Metals which can be drawn into thin wires are said to be ductile
In-text questions set 2 Page number 46
1. Why is sodium kept immersed in kerosene oil?

Solution:
The element sodium is reactive. It will react with oxygen if left open, exploring and catching fire. To stop sodium metal from reacting with oxygen, moisture, and carbon dioxide in the air, it is maintained submerged in kerosene.2. Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
Solution: (i) Iron reacts with steam to form a magnetic oxide of Fe with the liberation of H 2 .
3Fe(s) + 4H 2 O(g) → Fe 3 O 4 (s) + 4H 2 (g)(ii) Calcium reacts with water to form calcium hydroxide and hydrogen.
Ca(s) + 2H 2 O(I) → Ca(OH) 2 (aq) + H 2 (g) Potassium reacts with cold water violently immediately with evolution of H 2 which catches fire. 2K(s) + 2H 2 O(I) → 2KOH(aq) + 2H 2 (g)3. Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows
| Metal | Iron(II) sulphate | Copper(II) sulphate | Zinc sulphate | Silver Nitrate |
| A | No reaction | Displacement | – | – |
| B | Displacement | – | – | – |
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
Use the table above to answer the following questions about metals A, B, C and D.
- Which is the most reactive metal?
- What would you observe if B is added to a solution of Copper (II) sulphate?
- Arrange the metals A, B, C and D in the order of decreasing reactivity.
Solution:
(i) Metal B is the most reactive as it gives displacement reaction with iron (II) sulphate. (ii) A displacement reaction will occur when metal B is added to copper (II) sulphate solution, causing the solution's blue colour to disappear and forming a reddish-brown copper deposit on metal B. (iii) Because it removes iron from its salt solution, metal B is the most reactive. Because Metal A extracts copper from its salt solution, it is less reactive. Because metal C can only remove silver from its salt solution, it is nevertheless less reactive than metal D, which is least reactive as it cannot remove any metal from its salt solution. As a result, B > A > C > D is the decreasing order of the metals' reactivity.4. Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H 2 SO 4 .
Solution:
Hydrogen gas is liberated when dilute HCl is added to a reactive metal. Fe(s) + H 2 SO 4 (aq) → FeSO 4 (aq) + H 2 (g)5. What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Solution:
Compared to iron, zinc is more electropositive and reactive. Zinc so pushes out Iron from its salt solution. Ferrous sulphate is pale green in appearance, but it eventually becomes colourless. FeSO 4 + Zn → ZnSO 4 + Fe (s) Light green Zinc sulphate(Colourless)In-text questions set 3 Page number 49
1. (i) Write the electron-dot structures for sodium and oxygen.
(ii) Show the formation of Na 2 O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Solution: (i) Sodium:

Oxygen:

(ii) Formation of Magnesium oxide:
The two outermost electrons of a magnesium atom are transferred to an oxygen atom during a reaction with oxygen. The magnesium atoms produce a magnesium ion (Mg2+) by losing two electrons, and the oxygen atom makes an oxide ion (O2-) by receiving two electrons. Mg: + → MgO
→ MgO
Formation of Sodium oxide:
An oxygen atom receives the two outermost electrons from two sodium atoms. The two sodium atoms combine to generate sodium ions (2Na+) by losing two electrons. Additionally, the oxygen atom gains two electrons to create an oxide ion (O2-).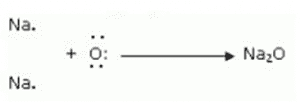
(iii) The ions present in sodium oxide compound (Na 2 O) are sodium ions (2Na + ) and oxide ions (O 2- ).
The ions present in Magnesium oxide compound (MgO) are magnesium ions Mg 2+ and oxide ions (O 2- ).2. Why do ionic compounds have high melting points?
Solution:
Compounds with both positive and negative charges are known as ionic compounds. As a result, they will be strongly attracted to one another. Ionic compounds have high melting points because it takes a lot of heat to overcome this force of attraction.In-text questions set 4 Page number 53
1. Define the following terms.
(i) Mineral
(ii) Ore
(iii) Gangue
Solution:
- Compounds, sometimes referred to as elements, are naturally occurring substances that form part of the earth's crust. For instance, K2SO4 and Alums.3.24 H2O in Al2(SO4), etc.
- Metal can be mined from minerals called ores. For example, bauxite Al2O3.The ore of aluminium is 2H2O, copper pyrite, or CuFeS2. While all ores are also minerals, not all minerals are regarded as ores.
- Sand and other stony elements naturally contaminate ores that are mined from the earth. The term "gangue" refers to impurities found in the ore.
2. Name two metals which are found in nature in the free state
Solution: Gold and platinum are the two metals found in free state in nature.
3. What chemical process is used for obtaining a metal from its oxide?
Reduction method is used to obtain metal from its oxide. Ex: Zinc oxide is reduced to metallic zinc by heating with carbon. ZnO + C → Zn + CO Ex: Lead oxide is reduced to lead by heating with carbon PbO +C → Pb + COIn-text questions set 5 Page number 55
1. Metallic oxides of zinc, magnesium and copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
| Zinc Oxide | |||
| Magnesium Oxide | |||
| Copper Oxide |
Solution:
A less reactive metal may be driven out of its oxide by a more reactive metal. Magnesium is the most reactive metal among zinc, magnesium is the least reactive, and zinc is the least reactive.The following situations will result in the displacement reaction.| Metal | Zinc | Magnesium | Copper |
| Zinc Oxide | – | Displacement | – |
| Magnesium Oxide | – | – | – |
| Copper Oxide | Displacement | Displacement | – |
2. Which metals do not corrode easily?
Solution: Gold and platinum are the metals which do not corrode easily
3. What are alloys?
Solution: An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal.
Exercise questions Page number 56-57
1. Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl 2 solution and aluminium metal
(c) FeSO 4 solution and silver metal
(d) AgNO 3 solution and copper metal
Solution:
The right response is option d, which calls for copper and AgNO3 solution. In the process of dislodging the silver cations and reducing them to the elemental metal, copper oxidises to form Copper II cations (Cu2+) and dissolves into solution. Consequently, a copper II nitrate solution will persist and silver metal can be seen precipitating out. Cu(s) + 2AgNO 3 (aq) → Cu(NO 3 ) 2 (aq) + 2Ag (s)2. Which of the following methods is suitable for preventing an iron frying pan from rusting?
- Applying grease
- Applying paint
- Applying a coating of zinc
- All of the above
Solution: Answer is (c) Applying a coat of zinc
While painting and using grease can stop iron from rusting, these techniques are inapplicable to frying pans. Therefore, the best way to stop an iron pan from rusting is to put a layer of zinc.3. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) Calcium
(b) Carbon
(c) Silicon
(d) Iron
Solution: Correct answer is option (a) Calcium.
Calcium oxide is produced when calcium and oxygen combine. When calcium oxide is dissolved in water, calcium hydroxide is produced. Option B is incorrect since carbon and oxygen combine to generate carbon-oxide, which is a gas. Silicon dioxide is created when silicon and oxygen combine. In water, this is insoluble. Option C is therefore incorrect. Iron dioxide is created when iron and oxygen combine. In water, this is insoluble. Option D is therefore incorrect.4. Food cans are coated with tin and not with zinc because
(a) Zinc is costlier than tin.
(b) Zinc has a higher melting point than tin.
(c) Zinc is more reactive than tin.
(d) Zinc is less reactive than tin.
Solution: Answer is c. Food cans are coated with tin and not with zinc because zinc is more reactive, that is electro positive than tin.
5. You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Solution:
Because they are pliable, metals may be readily hammered into sheets. However, non-metals decompose upon being beaten and cannot be consolidated into sheets due to their non-malleability. Because metals are excellent electrical conductors, connecting metals to wire, battery, and lightbulb results in a bulb. In a similar vein, non-metals that are poor electrical conductors do not light up the lightbulb when connected to the wire and battery. These experiments can be useful in demonstrating the electric conductivity and malleability of both metals and non-metals.6. What are amphoteric oxides? Give two examples of amphoteric oxides.
Solution:
Amphoteric oxides are oxides that react with bases and acids to produce water and salt. Al2O3 and PbO are two examples. Amphoteric oxides are the ones that combine with bases and acids to generate water and salt. Lead oxide (PbO) and aluminium oxide (Al2O3) are two examples.7. Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Solution:
Due to their extreme reactivity, magnesium (Mg) and zinc (Zn) are the two metals that will replace hydrogen in diluted acids. Because they are less reactive than hydrogen from diluted acids, gold (Au) and silver (Ag) cannot replace hydrogen.8. In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Solution:
An impure and thick block of metal M is used as the anode in the electrolytic refining process, while a thin strip or wire of pure metal M is used as the cathode. An appropriate metal M salt solution is regarded as the electrolyte.9. Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.

Solution: a) When sulphur powder is burnt in the air sulphur-di-oxide is formed.
(i) Sulphur-di-oxide does not have any effect on dry litmus paper. (ii) Sulphur-di-oxide turn the moist litmus paper from blue to red as contact of SO 2 with water turns to sulfurous acid.(b) S(s) + O 2 (g) → SO 2 (g)
SO 2 (g) + H 2 O →H 2 SO 310. State two ways to prevent the rusting of iron.
Solution:
Applying rust-resistant paint to the iron's surface will stop it from rusting. By using oil or grease on the surface of iron objects, you can keep the surface from coming into touch with wet air.11. What type of oxides are formed when non-metals combine with oxygen?
Solution: When non-metals combine with oxygen they form either acidic or neutral oxides. Ex: N 2 O 5 or N 2 O 3 is an acidic oxide; CO is a neutral oxide.
12. Give reasons
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction
Solution:
(A) Because platinum, gold, and silver are extremely low reactive metals, they are utilised to produce jewellery. They are therefore unaffected by most chemicals, water, or air. These metals are highly resistant to corrosion, have a lot of lustre, and are naturally malleable and ductile. (B) Lithium, potassium, and sodium react with water easily and quickly to produce a lot of heat. A fire results from the reaction's evolution of hydrogen. In order to avoid coming into touch with water, they react with atmospheric moisture, or water droplets, when exposed to water. These metals are thus kept under oil for storage. (C) Aluminium oxide generates a nonreactive surface on its surface. This type of coating stops other substances from interacting with aluminium. Thus, aluminium is utilised to make kitchen utensils. (D) It is simple to reduce metal oxide to free metal. In addition, the carbonate and sulphide ores are converted to oxides before the metals can be extracted from them since it is simpler to extract metals from their oxides than from their carbonates or sulphides.13. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Solution:
Because the acids in lemon or tamarind dissolve the copper oxide or basic copper carbonate layer that is present on the surface of tarnished copper vessels, these sour substances are used to clean them. They gleam reddish-brown once more as a result. As a result, they work wonders at polishing copper pots.14. Differentiate between metal and non-metal on the basis of their chemical properties.
Solution:
| Metals | Non-metals |
| When metals are heated with oxygen, they form ionic oxides which are basic in nature and form bases on dissolving with water. This turn red litmus paper to blue. | When non-metals are heated with oxygen, they form covalent oxides which are acidic in nature, which form acid on dissolving with water. This turn blue litmus paper to red. |
| They are electro positive, lose electrons readily and become a positive ion. | They are electro negative, gain electrons and become negative ions. |
| Metals are lustrous. | Non-metals are non-lustrous; graphite is the exception |
| Reducing agents. | Good oxidising agents. |
| Metals are the good conductors of electricity and heat. | Non-metals are non-conductors of electricity and heat; graphite is the exception |
| All metals are solids except mercury. | Non-metals are in solid-liquid and gaseous states. |
15. A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Solution:
The Latin term for royal water is Aqua regia, and this is the solution that the goldsmith employed. It is a 3:1 blend of concentrated nitric acid and concentrated hydrochloric acid. Noble metals like platinum and gold can dissolve in aqua regia. They become lighter as the top layer of the dull gold decoration dissolves.16. Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Solution:
Because copper does not react with water or steam, unlike iron, which interacts with steam to corrode the tank, copper is used to create hot water tanks instead of steel, an alloy of iron.Benefits of NCERT Solutions for Class 10 Science Chapter 3
The NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals offer several key benefits for students:Clear Understanding of Concepts : The NCERT Solutions for Class 10 Science Chapter 3 explain complex topics like the properties of metals and non-metals, their reactions, and the processes of metal extraction in simple, easy-to-understand language, helping students grasp the concepts effectively.
Step-by-Step Solutions : The NCERT Solutions for Class 10 Science Chapter 3 are presented in a systematic manner, guiding students through each problem with detailed, step-by-step explanations, which helps in better comprehension.
Reinforces Learning : By solving the NCERT questions with the help of the solutions, students can reinforce their learning and ensure a strong understanding of the chapter’s key points.
Exam Preparation : The NCERT Solutions for Class 10 Science Chapter 3 align with the CBSE exam pattern, helping students practice the types of questions that are likely to appear in the exams. This improves their confidence and performance.
Clarifies Doubts : Students can use the NCERT Solutions for Class 10 Science Chapter 3 to clear doubts and verify their answers, ensuring they are on the right track.
NCERT Solutions for Class 10 Science Chapter 3 FAQs
What are NCERT Solutions for Class 10 Science Chapter 3?
How do NCERT Solutions for Class 10 Science Chapter 3 help in board exam preparation?
Are the NCERT Solutions for Class 10 Science Chapter 3 sufficient for exam preparation?
Where can I find the NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals?










