Photoelectric Effect
Modern Physics of Class 12
PHOTOELECTRIC EFFECT
The emission of electrons from a metallic surface when irradiated by electromagnetic radiation is called the phenomenon of photoelectric effect. The emitted electrons are called as photoelectrons.
|
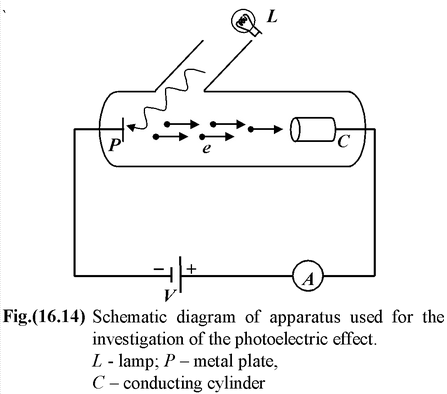
For the investigation of the photoelectric effect a schematic diagram of the apparatus as used by Lenord (1902) is shown in the fig.(16.14). Monochromatic light from the lamp L illuminates a plate P in an evacuated glass enclosure. A battery maintains a potential difference between P and a metal cylinder C, which collects the photoelectrons. The potential C can be varied to be either positive or negative relative to P. When the collector is positive with respect to the plate, the electrons are attracted to it and the ammeter (A) registers a current. Lenard studied the dependence of photoelectric current on the following factors. |
|
(i) Intensity of incident radiation
(ii) Potential difference between the plate and the collector
(iii) Frequency of the incident radiation
The result of observations are as follows:
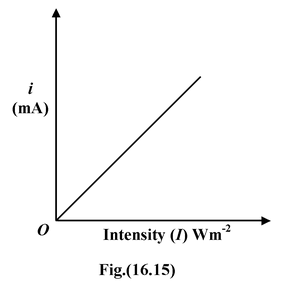
Effect of the intensity of Incident RadiationWhen the collector is positive relative to the plate and the potential difference is kept fixed, then for a given frequency of radiation, the photoelectric current is proportional to the intensity of the light, as shown in fig.(16.15). It shows that the number of emitted photoelectrons is proportional to the light intensity. Furthermore, there is no threshold intensity. |
|
Effect of Potential Difference
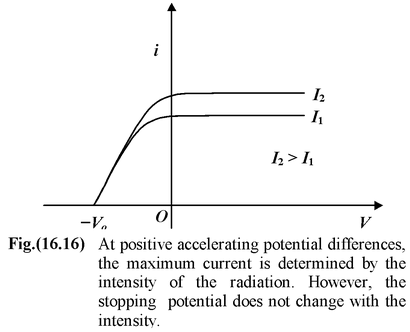
When the frequency and intensity of radiation are kept constant and the positive potential of collector relative to plate is gradually increased, then the photoelectric current i increases with the potential difference V. At some value of the potential difference, when all the emitted electrons are collected, thus increasing potential difference has no effect on the current. The current has reached its maximum value, called the saturation current.
When the polarity of the battery is reversed, the electrons are repelled and only the most energetic ones reach the collector, so the current falls. When the retarding potential difference reaches a critical value, the current drops to zero. At this stopping potential V o , only those electrons with the maximum kinetic energy are able to reach the collector.
eV o = 1/2 mv 2 max (16.29)
|
For a given frequency of light, the saturation current depends on the intensity of light. Larger the intensity; higher the saturation current. However, the stopping potential does not change with the intensity. It is clearly shown in fig. (16.16). Effect of frequencyFor a given intensity of radiation, the stopping potential depends on the frequency. Higher the frequency, higher the value of stopping potential. |
|
The maximum kinetic energy of the electrons depends on the light source and the plate material, but not on the intensity of the source. Certain combinations of light sources and plate materials exhibit no photoelectric effect.
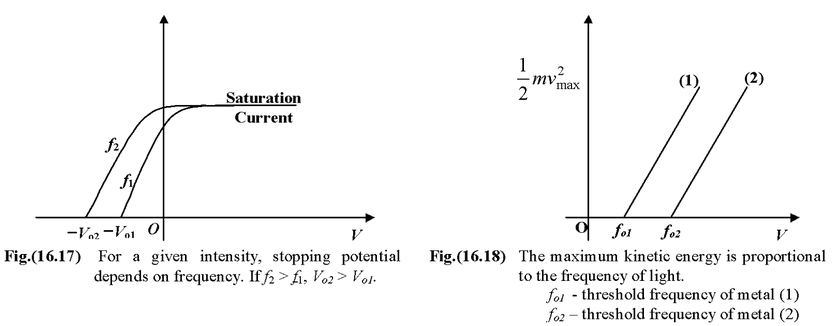
For a metal plate there exists a minimum frequency called threshold frequency (f o ) below which no electron is emitted however large the light intensity may be.
The threshold frequency is a characteristic of the metal plate.
Einstein’s Theory of Photoelectric Effect
According to Einstein, the experimental results of photoelectric effect can be explained by applying the quantum theory of light. He assumed that light of frequency f contain packets or quanta of energy E = hf. On this basis, light consists of particles, and these are called photons. The number of photons per unit area of cross-section of the beam of light per second is proportional to its intensity. But the energy of photon is proportional to its frequency and is independent of the light intensity.
In the process of photo emission a single photon gives up all its energy to a single electron. As a result, the electron is ejected instantaneously. Since the intensity of light is determined by the number of photons incident, therefore, increasing the intensity will increase the number of ejected electrons.
The maximum possible kinetic energy ( 1/2 mv 2 max ) of the photoelectrons is determined by the energy of each photon (hf) according to the Einstein equation (16.30)
1/2 mv 2 max = hf - W (16.30)
where the work function, (W), is the minimum energy needed to extract an electron from the surface of the material.
In terms of threshold frequency, it is given by
W = hf o (16.31)
Using equation (16.31), we may write equation (16.30) as
1/2 mv 2 max = hf – hf o = h(f – f o ) (16.32)
Also, in terms of stopping potential,
eV o = h(f – f o ) (16.33)
Example 16.22
Ultraviolet light of wavelength 2000Å causes photoemission from a surface. The stopping potential is 2V.
(a) Find the work function in eV
(b) Find the maximum speed of the photoelectrons.
Solution
(a) Using Einstein relation
W = hc/λ - eVo
or W = 12400/2000 - 2 = 4.2eV
(b) Since 1/2 mv 2 max = eVo
∴ v
max
=
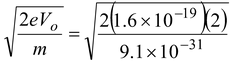
or v max = 8.4 × 10 5 m/s
How to determine the photoelectric current?
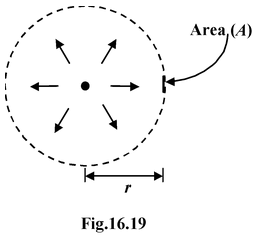
Let P be the power of a point source of electromagnetic radiations, then intensity I at
a distance r from the source is given by
I = P/4πr 2 (W/m 2 ) (16.34)
|
If A is the area of a metal surface on which radiations are incident, then the power received by the plate is P′ = IA = (P/4πr 2 )A (W) (16.35) If f is the frequency of radiation, then the energy of photon is given by E = hf |
|
The number of photons incident on the plate per second (called photon flux) is given by
Φ =
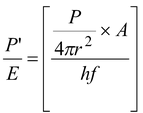 (16.36)
(16.36)
If f > f o (threshold frequency) and photon efficiency of the metal plate is η%, then the number of photoelectrons emitted per second is given by
n =
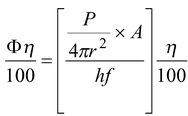 (16.37)
(16.37)
Finally, the photocurrent i is given by
i = ne (16.38)
where e is the charge of an electron (e = 1.6 × 10 -19 J)
Example 16.23
The intensity of sunlight on the surface of earth is 1400 W/m 2. Assuming the mean wavelength of sunlight to be 6000Å, calculate
(a) the photon flux arriving at 1 m 2 area on earth perpendicular to light radiations, and
(b) the number of photons emitted from the sun per second assuming the average radius of Earth’s orbit is 1.49 × 10 11 m.
Solution
(a) Energy of a photon E = hc/λ = 12400/6000 = 2.06eV = 3.3 x 10 -19 J
Photon flux = IA/E = (1400)(1)/3.3 x 10 -19 = 4.22 × 10 21
(b) n = P/E = I(4πR 2 )/E
or n = (1400)(4π)(1.49 x 10 11 ) 2 /3.3 x 10 -19 = 1.18 × 10 45