Ammonia Formula: Ammonia is an inorganic compound. Ammonia consists of nitrogen and hydrogen. It is a stable binary hydride and the simplest pictogen hydride. Ammonia is a colourless gas with a distinct pungent odour. Biologically it is a common nitrogenous waste and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilizers. Approx 70% of ammonia is used to make fertilisers in various forms and compositions like urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.
Ammonia, either directly or indirectly, is also a building block for the production of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
Ammonia is a chemical found in low quantities on Earth. It is produced from nitrogenous animal and vegetable matter. Ammonia and ammonium salts are also found in small quantities in rainwater, whereas ammonium chloride (sal ammoniac), and ammonium sulfate are found in volcanic districts. Crystals of ammonium bicarbonate have been found in Patagonia guano.
Ammonia Formula Symbol
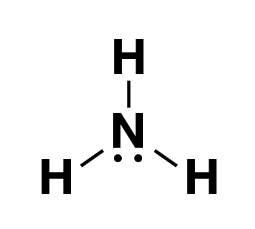
The chemical formula for ammonia is NH 3 . The symbol "NH 3 " represents the composition of one nitrogen (N) atom bonded to three hydrogen (H) atoms.
Ammonia Formula Structure
Ammonia formula is NH 3 . It is a combination of 1 Nitrogen atom and 3 Hydrogen atoms.The hydrogen atoms directly connected with Nitrogen with covalent bond.
Ammonia Formula Physical Properties
Below are some physical properties of Ammonia.
- Ammonia is a colourless gas.
- Ammonia is a strong and pungent odour.
- Ammonia gas solubility in water is 47% w/w at 0℃.
- Ammonia easily dissolves in Chloroform, Ethanol, Methanol, and Ether.
- Ammonia molar mass is 17.031 g/mol.
- Ammonia has a melting point of -77.73℃.
- Ammonia has a boiling point of -33.34℃.
Ammonia Formula Chemical Properties
Ammonia go through molecular autoionization and produce the base and conjugate. Below is the chemical reaction.
2NH 3 → NH 4 + + NH2 –
When concentrated ammonia reacts with concentrated hydrochloric acid. It produces clouds of Ammonia chloride. Below is the chemical reaction.
NH 3 + HCl →NH 4 Cl
Ammonia goes through combustion and produces nitrogen and water. Below is the chemical reaction.
4 NH 3 + 3 O 2 →2 N 2 + 6 H 2 O
4 NH 3 + 5 O 2 → 4 NO + 6 H 2 O
2 NO + O 2 → 2 NO 2
Liquid Ammonia reacts with lithium and produces lithium amide. Below is the chemical reaction.
2 NH 3 + 2 Li →2 LiNH 2 + H 2
Uses of Ammonia
Ammonia serves as a non-aqueous solvent for ionization purposes.
Ammonia serves as a Fertilizer.
Ammonia serves in many commercial cleaning products.
Ammonia is used in household products.
Ammonia serves for metal treatment
Ammonia serves as petroleum.
Ammonia serves as fuel in standard engines.
Ammonia serves as a building block material for the synthesis of pharmaceutical products.
Ammonia formula is NH 3 . It is an inorganic compound. Ammonia is a colourless gas known for its pungent odour. It consists of many chemical properties like autoionization , reaction with hydrochloric acid, combustion and production of lithium amide.
Ammonia finds important uses in agriculture as fertilizer. In the production of pharmaceuticals. It is used as solvent for ionization. It is used in many household and industrial cleaning products. It also plays a role in metal treatment, petroleum refining, and serves as a fuel for standard engines.
Ammonia is not only a synthetic compound but can also be naturally sourced from nitrogenous matter, small quantities in rainwater, volcanic regions, and even in the form of ammonium bicarbonate crystals. Its ubiquity and utility make it a crucial element in various facets of our daily lives and industries.
| Related Links | |
| Ammonium Hydroxide Formula | Strontium Sulfate Formula |
| Strontium Chloride Formula | Barium Bromide Formula |
Ammonia Formula FAQs
What is the chemical formula for ammonia?
What is the molecular structure of ammonia?
What are the physical properties of ammonia?
What are some common chemical properties of ammonia?
What are the primary uses of ammonia?