
As described by Linus Pauling, electronegativity is an element's ability to draw electrons towards itself. This property leaves atoms with a relative value compared to their bonded neighbors. Consequently, if one atom has a higher electronegativity than the other, the electron density of the bond will be skewed towards it, causing a slight negative charge on the more electronegative atom and a slight positive charge on the less electronegative one. For instance, if elements A and B are bonded together, and A has a greater electronegativity than B, then the electron pair of the bond will move towards A, making it slightly negatively charged. In contrast, element B becomes mildly positively charged.
These types of bonds are called polar bonds or polar covalent bonds. If the electronegativity of elements A and B is equal, they form a normal shared covalent bond. When the electronegativity difference between A and B is too high, A, being more electronegative than B, pulls both electrons towards itself completely, controlling both electrons completely. Irons are formed when A gets a negative charge, and B receives a positive charge. As a result, ionic bonds are formed between them.Periodic Trends in the Electronegativities of Elements
A period in the modern periodic table is characterized by an increasing nuclear charge and a decreasing atomic size as we move left to right. Therefore, the electronegativity of a period increases. Below is a chart depicting the electronegativity trend over period 3.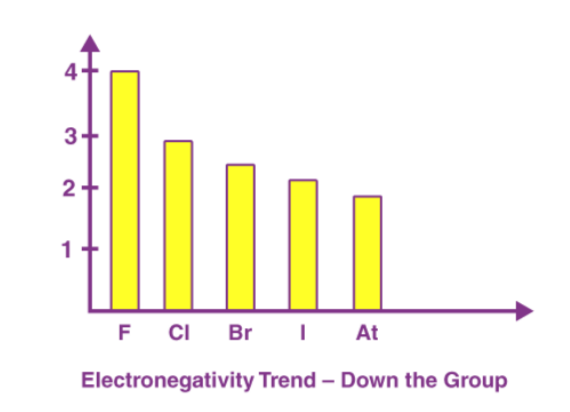 As we move down the modern periodic table, we see an increase in the atomic number, the nuclear charge also increases, but the effect of this increase in nuclear charge is offset by the addition of one shell. This leads to a decrease in electronegativity as we move down the group. It is shown in the diagram below that the electronegativity value decreases from fluorine to astatine in the halogen group.
As we move down the modern periodic table, we see an increase in the atomic number, the nuclear charge also increases, but the effect of this increase in nuclear charge is offset by the addition of one shell. This leads to a decrease in electronegativity as we move down the group. It is shown in the diagram below that the electronegativity value decreases from fluorine to astatine in the halogen group.
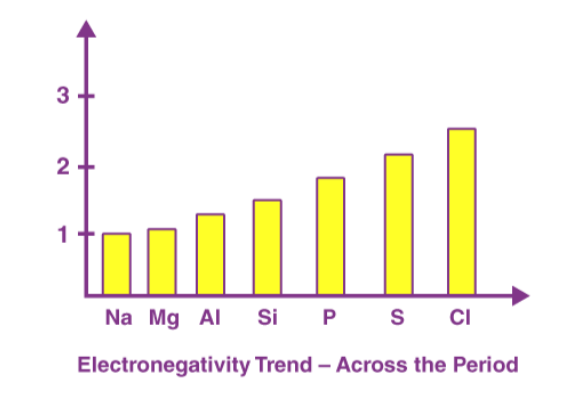 As a general rule, metals have a lower electronegativity than non-metals. In other words, metals are electropositive and non-metals are electronegative. Period two elements differ from their group elements in their properties due to their small size and higher electronegativity values.
As a general rule, metals have a lower electronegativity than non-metals. In other words, metals are electropositive and non-metals are electronegative. Period two elements differ from their group elements in their properties due to their small size and higher electronegativity values.
Also Read : Surface Chemistry Formula
A diagonal relationship is formed between the elements of the second period and those of the next group in period three because of a small difference in electronegativities.Most and Least Electronegative Elements
Fluorine is the most electronegative element on the periodic table. It has an electronegativity value of 3.98. Cesium has an electronegativity value of 0.79. Since electro positivity is the exact opposite of electronegativity, Cesium can be said to be the most electropositive element. Those elements with the fewest electrons required to complete their valence shells and the fewest inner electron shells between the positive nucleus and the valence electrons are the most electronegative. Fluorine is the most electronegative element. It has an electronegativity of 4.0. Metals have an electronegativity of less than 2.0. With electronegativity values of 0.7, cesium (Cs) and francium (Fr) are the least electronegative elements.Also Check - Value of Gas Constant Formula
As a result, The most electronegative element is fluorine, while the least electronegative element is cesium.Impact of Electronegativity on Covalent Bonding
The power of a covalent bond depends greatly on how electronegative the bonded atoms are, particularly the difference in their electronegativities. When dealing with homonuclear diatomic molecules, there is a 'pure' covalent bond since the two nuclei equally share the pair of electrons being held together. Examples of such bonds include H2, Cl2 and O2 molecules. On the other hand, a covalent bond between two species with different electronegativities tends to become polarized. In this process, the more electronegative atom pulls the electron pair closer to itself, developing a partially negative charge (denoted by the symbol -). The more electropositive atom is also characterized by a partial positive charge (denoted by +). These partial charges determine the chemical bond's polarity.Also Check - Boltzmann constant Formula
Bonds Between Highly Electronegative and Highly Electropositive Atoms
In covalent bonds featuring a large difference in the electronegativities of the bonded atoms, it is uncommon for the more electronegative atom to gain complete control over the bond pair of electrons, resulting in the formation of two ions. During the anion formation process, the more electronegative the atom, the more electropositive the atom. It is essential to be aware that all covalent bonds between different atoms contain a certain amount of ionic character. The reverse is true for ionic bonds too, as they will also have a slight covalent aspect. The difference between the electronegativities of each species can ascertain the degree of ionic character in the covalent bond. If this variation is minimal, then the link between them appears more covalent than ionic; if there is considerable disparity in electronegativity, the bond becomes predominantly polar and hence ionic.Electronegativity Table
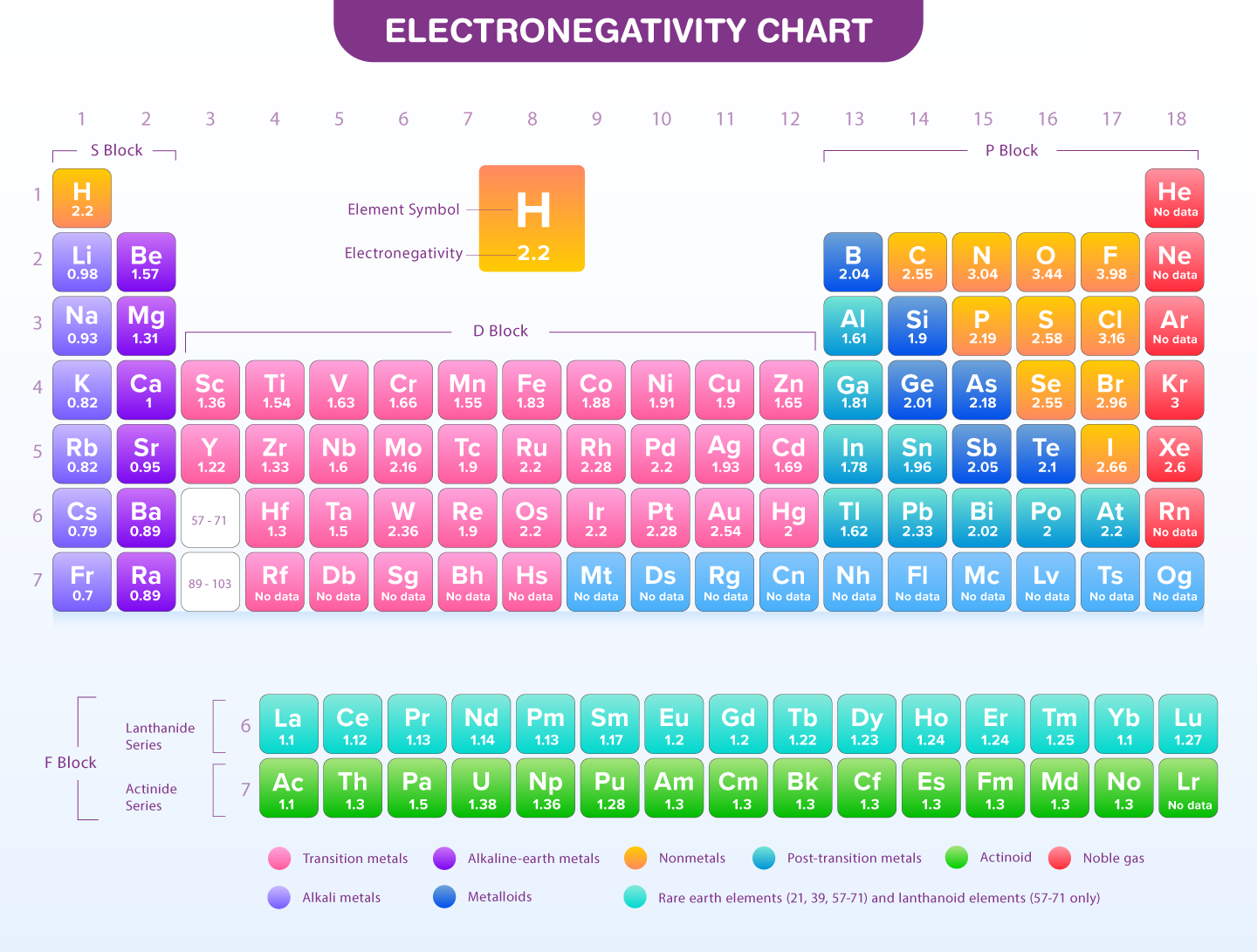 Chemical properties such as electronegativity describe the ability of an atom in a molecule to attract electrons that have been shared with another atom. Atoms on the left-hand side of the periodic table have a significant difference in electronegativity. An element's electronegativity will be considered as the primary factor in chemical bonding because it plays an important role in determining the nature of bonds between elements.
Chemical properties such as electronegativity describe the ability of an atom in a molecule to attract electrons that have been shared with another atom. Atoms on the left-hand side of the periodic table have a significant difference in electronegativity. An element's electronegativity will be considered as the primary factor in chemical bonding because it plays an important role in determining the nature of bonds between elements.
Also Check - Modern Periodic Table Formula
Factors Affecting Electronegativity
The size of an atom Due to electrons being further away from the nucleus, a larger atomic size will result in less electronegativity. Nuclear Charge Increasing nuclear charge causes electrons to attract with a greater force, which results in a greater electronegativity. Effect of Subsituent The electronegativity of an atom depends on the nature of the substitute attached to that atom. For example, the carbon atom in CF3I acquires a greater positive charge than CH3I. Therefore, the C atom in CF3I is more electronegative than in CH3I. Substitutes change an atom's electronegativity, which affects its chemical behavior.Electronegativity Formula FAQs
What is electronegativity?
How is electronegativity determined?
What is the significance of electronegativity in chemical bonding?
Which element has the highest electronegativity?
How does electronegativity affect molecule polarity?










