
Grams to Moles Formula: In chemistry, we often use moles to measure weight. Moles are a way to connect the weight of a substance to the number of its tiny building blocks, like atoms or molecules. For instance, one mole of something always contains around 6.022 × 10^23 of those building blocks. We use a formula to switch between grams and moles when we need to, depending on the substance's atomic weight.
Grams to Moles Formula
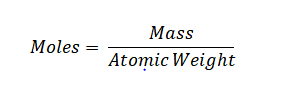
Also Check - Vapor Pressure Formula
Example 1: Converting 56 grams of water into moles.
Solution:
Given that the mass of water is 56 grams, we must use the molecular mass for this calculation since water is a molecule.
The molecular mass of water is 18.015 grams per mole.
Using the grams to moles conversion equation:
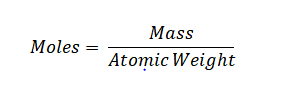
Moles = 56/18.015
Moles ≈ 3.10 moles
Also Check - Theoretical Yield Formula
Example 2: Converting 30 grams of oxygen into moles.
Solution:
Given that the mass of oxygen is 30 grams,
Oxygen has an atomic weight of 16 grams per mole.
Using the grams to moles conversion equation:
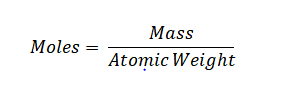
Moles = 30/16
Moles ≈ 1.875 moles
Also Check - Degree Of Unsaturation Formula
Example 3: Convert 40g of carbon dioxide (CO2) into moles?
Solution:
Given that the mass of carbon dioxide is 40 grams.
The molecular mass of carbon dioxide (CO2) is approximately 44.01 grams per mole.
Using the grams to moles conversion equation:
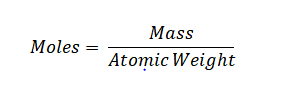
Moles = 40/44.01
Moles ≈ 0.909 moles
Also Check - Dilution Formula
Example 4: Convert 25g of sodium chloride (NaCl) into moles?
Solution:
Given that the mass of sodium chloride (NaCl) is 25 grams.
The approximate molar mass of sodium chloride (NaCl) is 58.44 grams per mole.
Using the grams to moles conversion equation:
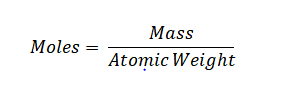
Moles = 25/58.44
Moles ≈ 0.428 moles
Also Check - Gas Pressure Formula
Example 5: Convert 72g of sulfuric acid (H2SO4) into moles?
Solution:
Given that the mass of sulfuric acid (H2SO4) is 72 grams.
The molar mass of sulfuric acid (H2SO4) is roughly 98.08 grams per mole.
Using the grams to moles conversion equation:
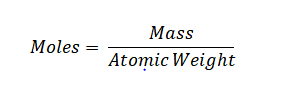
Moles = 72/98.08
Moles ≈ 0.734 moles
Example 6: Convert 60 grams of methane (CH4) into moles.
Solution:
In this problem, we are given the mass of methane, which is 60 grams. To convert these grams into moles, we need to use the molecular mass of methane.
The molecular formula of methane (CH4) indicates it consists of one carbon (C) atom and four hydrogen (H) atoms. To determine the molecular mass of methane, we calculate it by summing the individual atomic masses:
Carbon (C) has an atomic mass of approximately 12.01 grams per mole.
Hydrogen (H) has an atomic mass of approximately 1.01 grams per mole.
Therefore, the molecular mass of methane (CH4) can be calculated as:
Molecular mass = (1 × 12.01) + (4 × 1.01) = 12.01 + 4.04 = 16.05 grams per mole.
Now, we can use the grams to moles conversion equation:
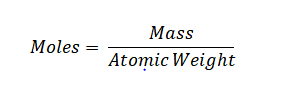
Moles = Mass (grams) / Molecular mass
Moles = 60 grams / 16.05 grams per mole
Moles ≈ 3.74 moles
Therefore, 60 grams of methane is approximately equal to 3.74 moles of methane.
Example 7: Convert 90 grams of calcium carbonate (CaCO3) into moles.
Solution:
In this problem, we have 90 grams of calcium carbonate (CaCO3) that we want to convert into moles. Calcium carbonate is a compound consisting of one calcium (Ca) atom, one carbon (C) atom, and three oxygen (O) atoms.
To find the molecular mass of calcium carbonate, we add up the individual atomic masses:
Calcium (Ca) has an atomic mass of approximately 40.08 grams per mole.
Carbon (C) has an atomic mass of approximately 12.01 grams per mole.
The atomic mass of oxygen (O) is approximately 16.00 grams per mole.
So, the molecular mass of calcium carbonate (CaCO3) is:
Molecular mass = (1 × 40.08) + (1 × 12.01) + (3 × 16.00) = 40.08 + 12.01 + 48.00 = 100.09 grams per mole.
Now, we can use the grams to moles conversion equation:
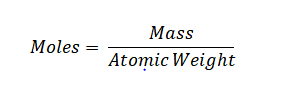
Moles = Mass (grams) / Molecular mass
Moles = 90 grams / 100.09 grams per mole
Moles ≈ 0.899 moles
Therefore, 90 grams of calcium carbonate is approximately equal to 0.899 moles of calcium carbonate.
These examples demonstrate how to convert the mass of a substance in grams into moles using the appropriate molecular or atomic mass, depending on whether the substance is a molecule or an element.
