
Sodium Nitrite Formula: Sodium, denoted by the symbol Na and having an atomic number of 11, is positioned in the inaugural group of the periodic table, alongside lithium and potassium. This element is characterized by its soft, silvery-white appearance and high reactivity, rendering it both flammable and a member of the alkali metal family. Sodium serves as an electrolyte crucial for maintaining water balance within and around cellular structures, and it plays a vital role in ensuring normal muscle and nerve functions.
Nitrogen, symbolized as N and possessing an atomic number of 7, falls within the 15th group of the periodic table as a nonmetal, representing the lightest member. It presents as a colorless, odorless, and tasteless element. Nitrogen claims the distinction of being the most abundant constituent in Earth's atmosphere, constituting approximately 78 percent of its composition. Moreover, nitrogen holds significant importance in the chemical industry, finding versatile applications in the production of fertilizers, nitric acid, nylon, dyes, and other essential compounds.
Also Check – Percent by Weight Formula
Sodium Nitrite
Sodium nitrite, classified as an inorganic compound, presents itself as a white to slightly yellowish crystalline powder characterized by its high solubility in water. This compound serves various purposes, primarily as a food preservative and as an antidote for cyanide poisoning. It's important to note that sodium nitrite shares similarities in name and application with sodium nitrate, as both are employed as preservatives in processed meat products like salami, hot dogs, and bacon. The effectiveness of sodium nitrite as a preservative stems from its potent oxidizing properties, which inhibit bacterial growth and colonization in food.
Also Check – Partial Pressure Formula
Sodium Nitrite Formula
The chemical composition of sodium nitrite comprises a sodium cation (Na + ) and a nitrite anion (NO 2 – ). To derive its formula, we refer to the periodic table, where sodium, represented by the symbol Na with an atomic number of 11, is classified as a metal. Conversely, the nitrite ion NO 2 – consists of nonmetals. Sodium, belonging to Group I, carries a 1+ ionic charge (Na +1 ), while the nitrite ion bears a 1- charge (NO 2 – ). Thus, the formula for sodium nitrite is accurately expressed as NaNO 2 , signifying its inorganic nature.
Also Check – Net Ionic Formula
Sodium Nitrite Molecular Structure
The structure of sodium nitrite involves the linkage of a sodium atom from the alkali group to the nitrite anion. When we examine this structure using Lewis notation, it becomes evident that the nitrite anion exhibits greater stability. Within this nitrite anion, it's important to note the presence of two potential resonance hybrid structures. Additionally, it's worth mentioning that the nitrite anion includes atoms that can possess formal charges.
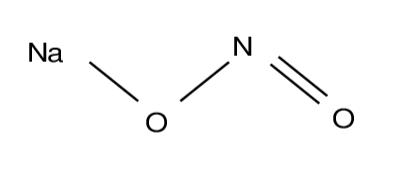
Preparation of Sodium Nitrite
There are several methods for producing sodium nitrite. One approach involves the reaction of nitrous acid with sodium hydroxide: 2 NaOH + N 2 O 3 ⇢ 2 NaNO 2 + H 2 O Sodium nitrite can also be obtained through the thermal reduction of sodium nitrate with calcium sulfite: NaNO 3 + CaSO 3 ⇢ NaNO 2 + CaSO 4 Another method entails heating sodium nitrate with carbon in the presence of either sodium hydroxide or calcium hydroxide: 2 NaNO 3 + 2 NaOH + C ⇢ 2 NaNO 2 + Na 2 CO 3 + H 2 O 2 NaNO 3 + Ca(OH) 2 + C ⇢ 2 NaNO 2 + CaCO 3 + H 2 OPhysical Properties of Sodium Nitrite
- Molecular Weight: 68.9953 g/mol
- Density: 2.17 g/cm3
- Boiling Point: 115°C
- Melting Point: 271°C
Chemical Properties of Sodium Nitrite
Chemical Formula: NaNO 2 Sodium nitrite is a relatively weak oxidizing agent. In an aqueous solution, sodium nitrite can react with sodium chloroacetate to produce nitromethane: NaNO 2 + ClCH 2 COONa + H 2 O ⇢ CH 3 NO 2 + NaCl + NaHCO 3Also Check – Internal Energy Formula
Side Effects of Sodium Nitrite
Sodium nitrite can pose risks when inhaled or absorbed through the skin, leading to several potential health concerns:
Gastrointestinal Distress: Consumption or exposure to sodium nitrite can result in symptoms like nausea, diarrhea, and abdominal pain.
Skin and Eye Irritation: Contact with sodium nitrite may irritate the skin and eyes, causing discomfort and redness.
Neurological Effects: Sodium nitrite exposure can lead to headaches, fatigue, dizziness, and even a bluish discoloration of the lips and skin.
Respiratory Irritation: Inhalation of sodium nitrite can irritate the nose and throat, causing respiratory discomfort.
Severe Effects at High Exposure Levels: In cases of extremely high levels of exposure, sodium nitrite can lead to severe breathing difficulties and, in extreme cases, even fatalities.
Safety Guidelines for Handling Sodium Nitrite
When working with Sodium Nitrite, it is crucial to adhere to the following safety measures:
Familiarize Yourself with Safety Instructions: Make sure to read and fully comprehend all safety precautions associated with Sodium Nitrite.
Prevent Environmental Release: Take precautions to prevent any unintended release of Sodium Nitrite into the environment.
Protect Your Eyes and Skin: Avoid direct contact with Sodium Nitrite by wearing appropriate protective gear such as gloves and safety glasses.
Thorough Handwashing: After handling Sodium Nitrite, thoroughly wash your hands to remove any residue.
No Eating, Drinking, or Smoking: While using Sodium Nitrite, refrain from eating, drinking, or smoking to prevent any accidental ingestion.
Keep Out of Reach: Ensure that Sodium Nitrite is stored in a location that is inaccessible to children and pets.
Uses of Sodium Nitrite
Sodium Nitrite finds various applications, including:
Food Preservation and Cyanide Antidote: It serves as a food preservative and is employed as an antidote for cyanide poisoning.
Preservation of Cured Meats: Sodium Nitrite is a common preservative used in the curing process of meat products.
Wild Boar Control: It is used as a toxin for controlling wild boar populations.
Enhancement of Meat Color: Sodium Nitrite contributes to the desirable red coloration in meat products.
Sodium Nitrite Formula FAQs
What is the chemical formula of Sodium Nitrite?
What does the formula NaNO2 represent?
What is the molecular weight of Sodium Nitrite?
Is Sodium Nitrite a strong oxidizing agent?










