Hydroxy Compounds (Alcohols)
Alcohol Phenol And Ether of Class 12
|
Monohydric i.e. |
|
|
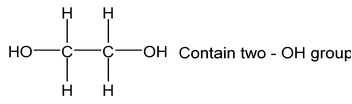
Dihydric |
|
|
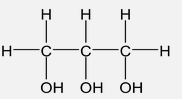
Polyhydric |
|
Contain three and more than three hydroxyl group.
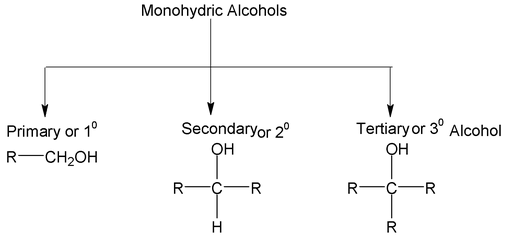
Monohydric alcohols are of three types.
PREPARATION OF ALCOHOLS
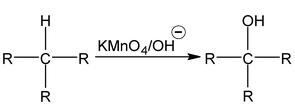
1. From Alkanes
Alkanes having tertiary carbon on oxidation with cold alkaline KMnO 4 give tertiary alcohol.
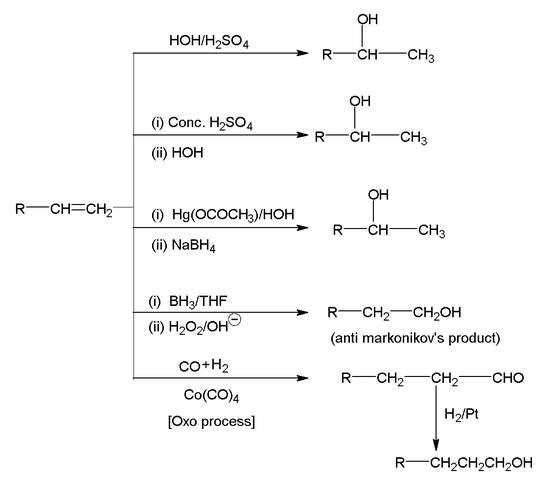
2. From Alkenes
Alkenes can be converted into alcohol by the following reactions:
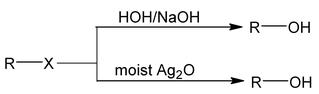
3. From alkyl halides
Alkyl halides give alcohol with KOH/NaOH or with moist Ag 2 O.
4. Reduction of aldehydes and ketones
(a) Reduction by reducing agents
(i) Aldehyde gives primary alcohol

(ii) Ketone gives secondary alcohol
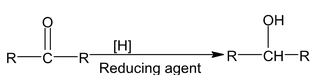
Reducing agents
(ii) LiAlH 4 (ii) NaBH4
(iii) Na/C 2 H 5 OH (iv) Metal (Zn, Fe or Sn)/Acid (HCl, dil H 2 SO 4 or CH 3 COOH)
(v) (a) Aluminium isopropoxide/isoproppylalcohol (b) H2O
(vi) H 2 /Ni
NaBH 4 and aluminium isopropoxide reduces only carbonyl group and has no effect on any other group.

Reducing with aluminium isopropoxides is known as Meerwein – Ponndorf Verley (MPV) reduction.
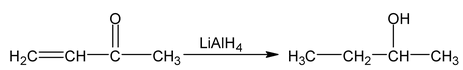
LiAlH 4 has no effect on double and triple bonds but if compound is β - aryl, α, β - unsaturated carbonyl compound then double bond also undergoes reduction.

(b) Reduction by Grignard reagents
Addition followed by hydrolysis
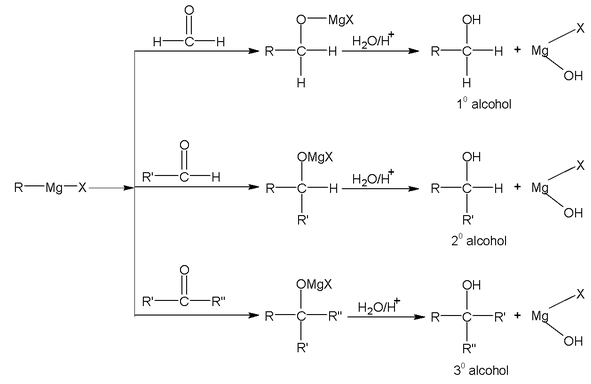
Methanol can not be prepared by this method.
5. Reduction of carboxylic acid, Acid chlorides and esters:
(a) Reduction by LiAlH 4
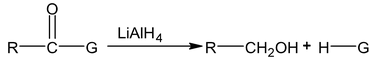
G = OH (acid)
G = Cl (acid chloride)
G = OR′ (ester)
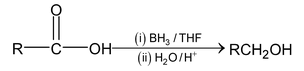
(b) Reduction by BH 3
Carboxylic acids and esters are reduced in to primary alcohol by BH 3 .
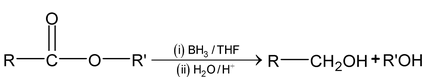
(c) Bouveault – Blanc reaction
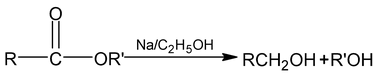
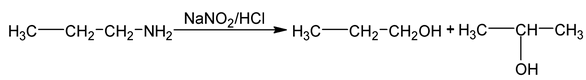
6. From aliphatic primary amines
It react with nitrous acid to give alcohol.
Nature of alcohol depends on the nature of carbon having ⎯NH 2 group.
Reaction proceeds through carbocation hence rearranged alcohol is obtained.
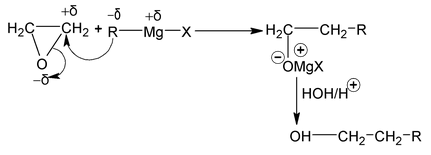
7. From Oxiranes
Oxiranes react with Grignard reagent to give mono hydric alcohol. Nature of G.R is basic hence it attack on less hindered carbon of oxirane ring.
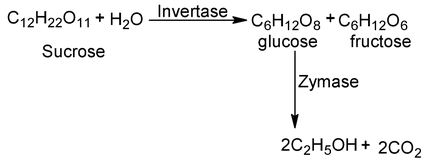
8. Fermentation of carbohydrates