
Soaps And Detergents
Carbon And Its Compound of Class 10
SOAPS AND DETERGENTS
The word ‘Detergent’ means cleaning agent and so the detergents are substances which remove dirt and have cleansing action in water. According to this definition of detergents, soap is also a detergent and has been used for more than two thousand years.
There are two types of detergents:
(i) Soapy detergents or soaps
(ii) Non-soapy detergents or soapless soap.
SOAP:
A soap is a sodium or potassium salt of some long chain carboxylic acids (fatty acid). Sodium salts of fatty acids are known as hard soaps and potassium salts of fatty acid are known as soft soaps. Soap has a large non-ionic hydrocarbon group and an ionic COO – Na + group. The structure of soap can be represented as:
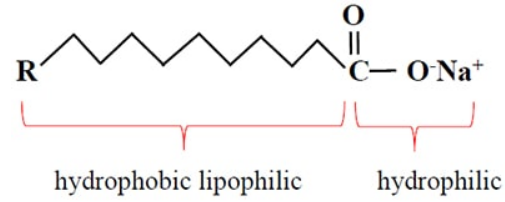
Where represents the hydrocarbon group and ! represents negatively charged carboxyl group. Some examples of soaps are sodium stearate, C 17 H 35 COO – Na + , sodium palmitate, C 15 H 31 COO – Na + and sodium oleate, C 17 H 33 COO – Na + .
|
|
Hard water, which contains salts of magnesium and calcium, reacts with soap to form magnesium and calcium salts of fatty acid. |
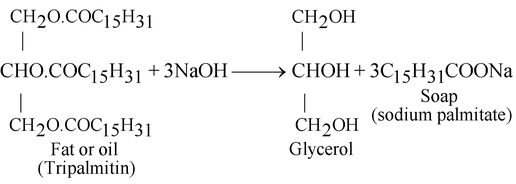
Preparation of soap:
Soap is prepared by heating oil or fat of vegetable or animal origin with concentrated sodium hydroxide solution (caustic soda solution). Hydrolysis of fat takes place and a mixture of sodium salt of fatty acids thus formed are used as soap so alkaline hydrolysis of oils and fats is commonly known as saponification.
(ii) Limitation of soap:
Soap is not suitable for washing clothes with hard water because of the following reasons:
(a) Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions of hard water react with soap forming insoluble calcium and magnesium slats of fatty acids.

Therefore, a lot of soap is wasted if water is hard.
(b) When hard water is used, soap forms insoluble precipitates of calcium and magnesium salts, which stick to the cloth being washed. Therefore, it interferes with the cleaning ability of the soap and makes the cleaning process difficult.
|
|
These calcium and magnesium salts of fatty acids are insoluble in water and separate as curdy white precipitate. |
DETERGENTS:
Detergents are also called ‘soap-less soaps’ because though they act like a soap in having the cleansing properties, they do not contain the usual ‘soaps’ like sodium stearate, etc.
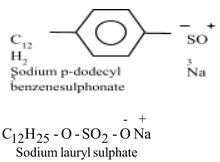
A detergent is the sodium salt of a long chain benzene sulphonic acid (or the sodium salt of a long chain alkyl hydrogensulphate) which has cleansing properties in water. A detergent has a large non-ionic hydrocarbon group and an ionic group like sulphonate group, SO3 – Na + , or sulphate group SO4 – Na + . Examples of detergents are : Sodium n- dodecyl benzene sulphonate and Sodium n-dodecyl sulphate. These are shown below :
The structure of a detergent is similar to that of soaps. A detergent molecule also consists of two parts : a long hydrocarbon chain which is water repelling (hydrophobic), and a short ionic part which is water attracting (hydrophilic).
Preparation of Synthetic Detergents:
Synthetic detergents are prepared by reacting hydrocarbons from petroleum with conc. Sulphuric acid and converting the product into its sodium salt.
Ex.:
|
|
|
COMPARISON BETWEEN PROPERTIES OF SOAPS AND DETERGENTS:
|
S.No. |
Soaps |
Synthetic Detergents |
|
1. |
Soaps are sodium slats of higher fatty acid |
Synthetic detergents are sodium alkyl sulphates or sodium alkyl benzene sulphonates with alkyl group having more than ten carbon atoms. |
|
2. |
Soaps are prepared from natural oils and fats. |
Synthetic detergents are prepared from the hydrocarbons of petroleum. |
|
3. |
Soaps form insoluble salts (curdy white ppt.) with calcium and magnesium which are present in hard water and hence, cannot be used in hard water. |
Calcium and magnesium salts of detergents are soluble in water and, therefore, no curdy white precipitates are obtained in hard water and hence, can be used even in hard water. |
|
4. |
Soaps cannot be used in acidic medium as they are decomposed into carboxylic acids in acidic medium. |
They can be used in acidic medium as they are the salt of strong acids and are not decomposed in a acidic medium |
|
5. |
Soaps are biodegradable. |
Some of the synthetic detergents are not biodegradable. |
ADVANTAGES OF SYNTHETIC DETERGENTS OVER SOAPS:
Synthetic detergents are widely used as cleaning agents these days. Some of their advantages over soaps are:
- Synthetic detergents can be used for washing even in hard water. On the other hand, soaps are not suitable for use with hard water.
- Synthetic detergents can be used even in acidic solutions because they are not readily decomposed in acidic medium. On the other hard, soaps cannot be used in acidic medium because they are decomposed into carboxylic acids in acidic medium.
- Synthetic detergents are more soluble in water than soaps.
- Synthetic detergents have a stronger cleaning action than soaps.
CLEANSING ACTION OF SOAPS AND DETERGENTS:
Both soaps and detergents are made up of two parts, i.e., a long hydrocarbon tail and a negatively charged head. The hydrocarbon tail being non-polar is insoluble in water and hence is hydrophobic(water repelling). On the other hand, the negatively charged head being polar is soluble in water and hence is hydrophilic(water-attracting).
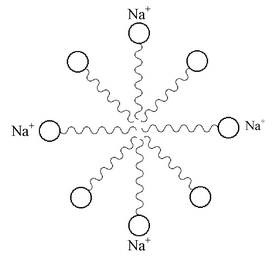
When a soap or a detergent is added to water the polar heads of their molecules dissolve in water while non-polar tails dissolve in each other. As a result, the soap or the detergent forms spherical ionic micelles, i.e., clusters of about 100-200 molecules with their polar heads (shown by solid circles) on the surface of the cluster and the non-polar chains (shown by wavy lines) directed towards the centre. In a similar way, detergents also form ionic micelles. These micelles remain suspended in water as a colloid and will not come together to precipitate out due to repulsion between the similar negative charges.
|
|
A spherical aggregate of soap molecule in soap solution in the water is called ‘micelle’. The soap micelles are quite large and hence they scatter light. That is why a soap solution appears cloudy. |
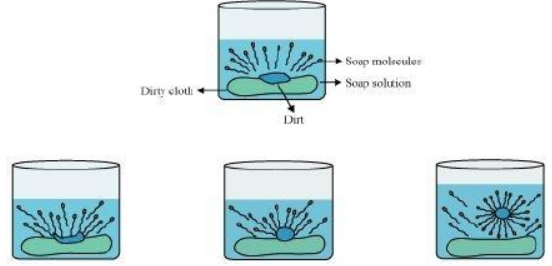
The dirt is generally held to the surface of a dirty cloth by a thin film of oil or grease. When a dirty cloth is treated with soap or detergent solution, the non-polar hydrocarbon tails of the soap or the detergent dissolve in oil or grease while the polar heads are held by the surrounding water. The stepwise formation of these micelles is shown in figure. In other words, soap or the detergent is attracted to both the greasy dirt and water. This lowers the surface tension of water and a stable emulsion of oil in water is formed.
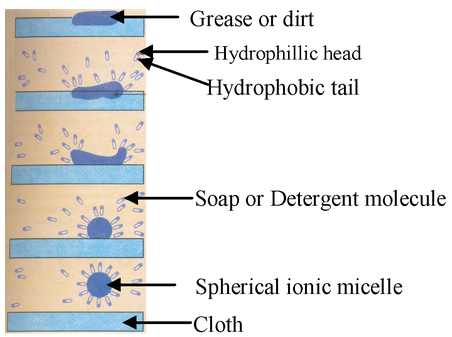
When the surface of the cloth is mechanically scrubbed or beaten on a stone or with a wooden paddle or agitated in a washing machine, the loosened oily dirt particles are removed from the dirty surface and the cloth is cleaned. Since detergents lower the surface tension of water to a greater extent than soaps, therefore, the cleansing power of detergents is much higher than those of soaps.
SYNTHETIC DETERGENTS: A SEROUS PROBLEM:
It may be noted that in the past, the widespread use of detergents caused pollution of rivers and other water bodies. Earlier the synthetic detergents were made from long chain of hydrocarbons having a lot of branched chains in them. These branched chain detergent molecules were degraded very slowly by the micro organisms present in water bodies like lakes or rivers.
Therefore, they tend to remain in water bodies for a long time and make water unfit for aquatic life. For example, detergents containing phosphates can cause rapid growth of algae and therefore, deplete the dissolved oxygen present in the water of lakes and rivers. As a result of lack of oxygen, fish and other aquatic animals may die.
To solve these problems, now-a-days, the detergents are prepared from hydrocarbons which have minimum branching. These are degraded more easily than branched chain detergents. Therefore, these are biodegradable and create fewer problems.
Solved questions
1. Why is carbon tetravalent ?
Solution: Carbon atom has 4 electrons in the outermost shell.It needs 4 more electrons to complete its octet. Therefore , carbon is tetravalent
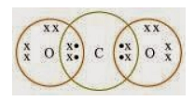
2. What would be the electron dot structure of carbon dioxide which has the formula CO 2 ?
Solution: Electron dot structure of CO 2 is
3. What is the valency of carbon in CH 3 -CH3, CH 2 =CH 2 and HC=CH ?
Solution: The valency of carbon in all its compounds whether saturated or unsaturated is 4.
4. Why is high temperature not favourable for alcoholic fermentation?
Solution: The high temperature destroys the enzymes which are needed to carry fermentation .
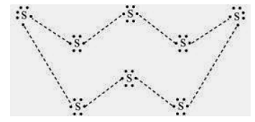
5. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint − the eight atoms of sulphur are joined together in the form of a ring.)
Sol. Electron dot structure of a sulphur molecule
6. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Sol. The two features of carbon that give rise to a large number of compounds are as follows:
(i) Catenation − It is the ability to form bonds with other atoms of carbon.
(ii) Tetravalency − With the valency of four, carbon is capable of bonding with four other atoms.
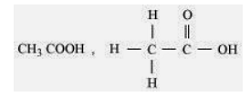
7. Draw the structures for the following compounds.
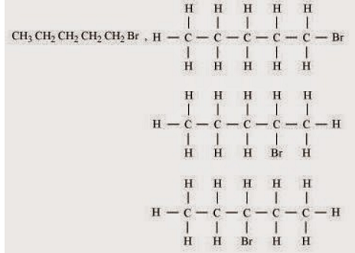
(i) Ethanoic acid (ii) Bromopentane*
(iii) Butanone (iv) Hexanal
*Are structural isomers possible for bromopentane?
Sol. (i)
(ii) There are many structural isomers possible for bromopentane. Among them, the structures of three isomers are given.
(iii)
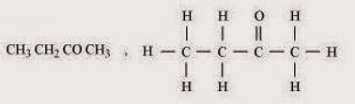
(iv)
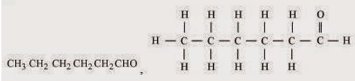
8. What is the difference in the molecular mass of any two adjacent homologues?
Solution: 14 mass units.
9. Which substance is added to denature ethyl alcohol?
Solution: A small amount of methyl alcohol , pyridine or copper sulphate is added to denature ethyl alcohol.
10. Which gas is evolved when sodium carbonate or bicarbonate is added to ethanoic acid ?
Solution: CO 2 (carbon dioxide).
11. Explain the formation of scum when hard water is treated with soap.
Solution: Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
12. What change will you observe if you test soap with litmus paper (red and blue)?
Solution: Since soap is basic in nature, it will turn red litmus blue. However, the colour of blue litmus will remain blue.
13. What is hydrogenation? What is its industrial application?
Solution: Hydrogenation is the process of addition of hydrogen. Unsaturated hydrocarbons are added with hydrogen in the presence of palladium and nickel catalysts to give saturated hydrocarbons.
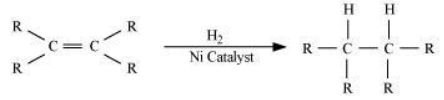
This reaction is applied in the hydrogenation of vegetables oils, which contain long chains of unsaturated carbons.
14. Explain the mechanism of the cleaning action of soaps.
Solution: Cleansing action of soaps:
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.
15. Give a test that can be used to differentiate chemically between butter and cooking oil.
Solution: Butter contains saturated fats. Therefore, it cannot be hydrogenated. On the other hand, oil has unsaturated fats. That is why it can be hydrogenated to saturated fats (solids).










