Dipole Moment In Aromatic Ring System
Chemical Bonding of Class 11
DIPOLE MOMENT IN AROMATIC RING SYSTEM
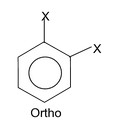
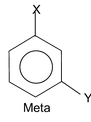
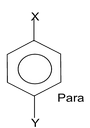
The dipole moments of the aromatic compounds present a very good illustration of dipole moments. We all know when substituted benzene is treated with a reagent different products namely ortho, meta and para products are formed. The dipole moments of these products are different since the orientation of the groups is different at ortho, meta and para position. Let us take an example which will make it easily digestive for you. Suppose we have three isomers of o-nitrophenol, m-nitrophenol and p-nitrophenol. We have also the e.g. o-aminophenol, m-aminophenol and p-aminophenol.
|
|
|
|
In the case X = Y, the para isomer becomes symmetrical and have zero dipole.
Now the obvious question that is peeping through your mind is that which isomer in which case has got higher dipole moment. The answer lies in the nature of the groups linked to the benzene ring. In nitrophenol groups one group is electron pushing and the other is electron withdrawing while in the second case both the groups attached are electron pushing. So depending on the nature of the groups attached one of the isomer, o, m or p has the largest dipole moment.
Case I: Now when X & Y are both electron pushing or electron withdrawing.
Suppose, bond dipole of C – X = μ1
And that of C – Y = μ2
Here we have assumed a sign of + when groups are electron pushing and–when groups are electron withdrawing. The net dipole is the resultant of two bond dipoles at different orientations.
When both X & Y are electron pushing or electron withdrawing.
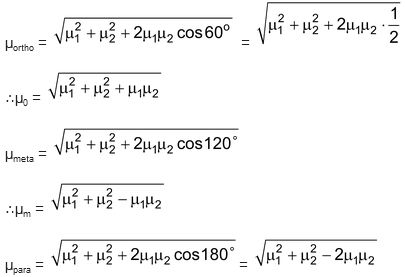
∴μp = μ1 – μ2
From the above expression of μ0, μm & μp it is clear that when both X & Y are of the same nature i.e. both are electron withdrawing or both are electron pushing the para product has the least dipole moment and ortho product has the highest. Now when X = Y, μp = μ1 – μ2 = μ1 – μ1 = 0
Which we have already discussed.
Case II: When X is electron pushing and Y is electron withdrawing or vice versa.
Let C – X dipole = μ1
& C – Y dipole = μ2
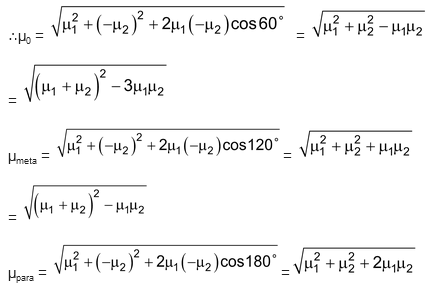
= μ1 +μ2
Now if you see the expressions, it is very clear that the para isomer has the highest dipole moment and ortho is the least.
So to calculate the dipole moments of disubstituted benzene one should consider about the nature of the groups linked and then only one can predict the dipole moment of the molecule.

Also Check