
Chemical effect of electric current
Chemical effect of electric current of Class 8
CHEMICAL EFFECT OF ELECTRIC CURRENT
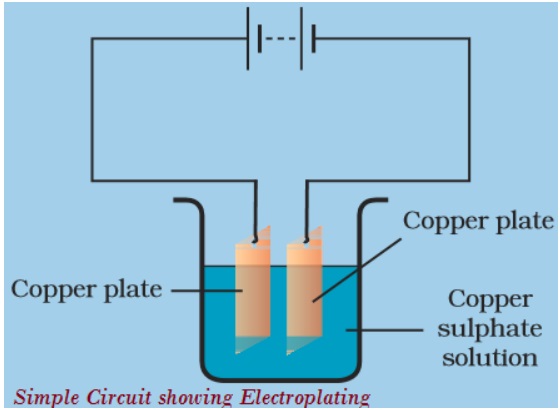
When an electric current is passed through water containing sulphuric acid, the water breaks up into its components hydrogen and oxygen. Therefore an electric current can cause a chemical change. This effect of electric current is used in electroplating i.e. coating a thin layer of a metal on another metal.The metal which is to be electroplated is made cathode and the metal to be deposited is made anode while the soluble salt of the same metal serves as the electrolyte. When a current is passed, a thin film of metal is deposited on the metal, which becomes electroplated.
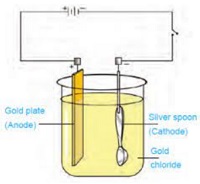
e.g. Let we are having a spoon which is to be electroplated with silver. The spoon is made the cathode and the piece of silver is made the anode. AgNO3 salt is used as electrolyte. When a current is passed through the electrolyte the atoms of silver gets deposited on the spoon.
GENERAL TERMS ASSOCIATED WITH THE PASSAGE OF CURRENT THROUGH SOLUTIONS:
(A) Electrolyte : A solution of a chemical compound which conducts electric current and at the same time undergoes a chemical change is called electrolyte.
e.g. (i) Aqueous solutions of all acids, such as HCI, HNO3, H2SO4 etc.
(ii) Aqueous solutions of all alkalis, such as NaOH, KOH etc.
(iii) Aqueous solutions of salts, such as common salt, copper sulphate, sodium nitrate, zinc chloride, etc.
(B) Non-electrolyte : A solution of a chemical compound which does not conduct electric current and hence does not undergo any chemical change is called non-electrolyte.
e.g. Petrol, kerosene oil, diesel oil, vegetable oil, chloroform, carbon tetrachloride, alcohol, ether, benzene, distilled water etc.
(C) Electrolysis : The process due to which a solution of a chemical compound conducts electric current and at the same time undergoes a chemical change is called electrolysis.
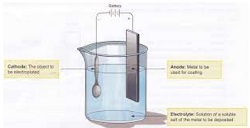
(D) Electrodes : The metal wires/plates/rods through which the current enters or leaves an electrolyte are called electrodes.
(E) Cathode : The electrode connected to the negative terminal of a cell/battery is called cathode.
(F) Anode : The electrode connected to the positive terminal of a cell/battery is called anode.
(G) Ions : The electrically charged atoms/group of atoms formed when a chemical compound is dissolved in water are called ions.
(H) Cations : The positively charged ions formed, when a chemical compound dissolves in water are called cations. During electrolysis, the cations are discharged at cathode by taking electric charges from it.
(I) Anions : The negatively charged ions formed, when a chemical compound dissolves in water are called anions, During electrolysis, the anions are discharged at anode by losing electric charges to it.
(J) Voltameter : An apparatus in which electrolysis is carried out, which consist of a vessel, two electrodes and electrolyte is called voltameter.
ELECTROPLATING :

One of the use of chemical effect of an electric current is electroplating. During electroplating the metal surface of a given particle is coated with a thin layer of superior metal with the help of electric current.
Electroplating is the application of a metal coating to a metallic or other conducting surface by an electrochemical process. The article to be plated (the work) is made the cathode (negative electrode) of an electrolysis cell through which a direct electric current is passesd. The article is immersed in an aqueous solution (the bath) containing the required metal in an oxidised form, either as an aquated cation or as a complex ion. The anode is usually a bar of the metal being plated. During electrolysis metal is deposited on to the work and metal from the bar dissolves:
at cathode M
z+(aq) + ze- → M(s)
at anode
M(s) → M(aq) + ze-
There are two main reasons for electroplating objects
- To protect the metal underneath;
- To produce an attractive finish.
Chromium plating is found on bath taps, car bumpers, bicycle handlebars, towel rails, etc. Chromium does not corrode. It can be polished to give a bright attractive appearance, and it is a hard metal which resists scratches and wear.
Faraday's laws of electrolysis govern the amount of metal deposited.
Articles are elecroplated to (i) alter their appearance; (ii) to provide a protective coating; (iii) to give the article special surface properties; (iv) to give the article engineering or mechanical properties.
Let’s find: How is thin layer of copper metal deposited on the given object :
The saturated copper sulphate solution contains the following cations and anions.
Cations : The positively charged cations are copper ions (Cu 2+ ) from copper sulphate and hydrogen ion (H + ) from water.
Anions: The negatively charged anions are hydroxyl ions (OH - ) from water and sulphate ions (SO 4 2- ) from copper sulphate.
When the electrical current is switched on, the cations start migrating towards the cathode and the anions towards the anode.
At the cathode the positively charged copper ions gain negative electrical charges to form copper atoms which deposit themselves on the surface of iron object. Thus, a thin layer of copper is deposited on iron objects.
The hydrogen ion do not get discharge. At anode, none of the negatively charged anions (hydroxyl and sulphate ions) discharge. Instead, the copper atoms on the copper plate lose their charges to form copper ions (Cu 2+ ) which enter in the copper sulphate solution.
Uses of electrolysis :
- Electroplating is one of the uses of electrolysis. It is not only used for depositing copper, but a number of superior metals. For example, the wheel covers of cars, the handles of bicycles and motorcycles are coated with nickel and chromium, so as to give a bright shining appearance. Similarly, silver and gold can be electoplated on copper and brass object. Cheap imitation jewellery is made by electroplating silver or gold on brass or aluminium jewellery.
- The process of eletrolysis is also used to obtain pure metals from impure metals.
- The process of electrolysis is also used in the extraction of aluminium metal from its ore.
THUNDER AND LIGHTNING
ELECTRIC CHARGE
From the study of atomic structure, we know that an atom consists of a central part called nucleus and around the nucleus (called extra-nucleus) there are a number of electrons revolving in different paths or orbits. The nucleus consist of protons and neutrons. A proton is a positively charged particle while a neutron has no charge. Therefore, the nucleus of an atom bears a positive charge. An electron is a negatively charged particle having magnitude of negative charge equal to the magnitude of positive charge on a proton. Normally, the number of electrons is equal to the number of protons in an atom. Therefore, an atom is neutral as a whole, the negative charge on electrons cancelling the positive charge on protons. This leads to the conclusion that under ordinary conditions, a body is neutral, i.e. it exhibits no charge. When a body has deficiency or excess of electrons from the normal, it is said to be charged or electrified.
TYPES OF CHARGE:
There are two types of charges known as positive and negative charges. All objects normally contain equal amount of positive and negative charges and are therefore, electrically neutral.
Eg. (i) When we comb dry hair, the comb gets charged and can pick small pieces of paper brought near it.
(ii) When we rub a glass rod with silk cloth or a piece of ebonite rod with woolen material. The charge acquired by a glass rod rubbed with silk is called a positive charge and that on ebonite rod is called a negative charge.
Glass rod and ebonite rod will attract each other while two glass rods as well as two ebonite rods will repel each other.
Like charges repel each other and unlike charges attract each other.
CHARGED CLOUDS
The clouds consists of electric charges in which the lower portion of clouds carry negative charges and the upper portion carry positive charges. This huge amount of charges makes the insulation property of air gets breakdown and the nearby air molecules get apart. Bythis, the air molecules are charged and the air containing charged particles become a conductor of electricity. The ripping apart of the air molecules occur in steps. The successive layers of air are made conductive in zig-zag or step like path
.

Step leader is the path of conductive air which extends from the thundercloud. Step leader form the conductive path in the air, from the cloud to the ground or to a neighbouring cloud. This step leader is not as bright as the flash of light.
LIGHTNING
We know that sometimes during rain, thunder and lightning also takes place. During rain over the sky flashes of light are also observe. This natural phenomenon is called lightning. During lightning strike, ten to twenty thousand amperes of electric current flows. The air in the path of lightning heats up and gets hotter than the surface of the sun (about 30,000°C). This causes the flash of lightning.

The thunder that we hear during rain is due to the wave of vibrations (shock wave) which occur due to enormous amount of heat produced and make the air expand suddenly.
Therefore, a lightning is a high-energy electric discharge accompanied by a large amount of heat and light. This can happen between a charged cloud and the ground, between two charged clouds or even between two oppositely charged portions of the same cloud.
Thunderstorms often occur at the end of hot, sticky summer days. At this time, warm moist air rises quickly and forms large cumulonimbus clouds. Inside these tall dark clouds, air currents create strong up draughts and water droplets and ice particles rub against each other. As they bang together like this, it causes a build-up of static electricity. Lighter, positive charges gather at the top of the cloud and heavier, negatively-charged pieces of ice and water accumulate at the base. The ground below is also positively charged. Electricity flows between the charges and the difference between them increases. When the differences get big enough, they are neutralized and electricity is released as a flash of lightning.For More practice go for chapter wise online test for class 8
FORMATION OF LIGHTNING:
The sky is filled with electric charge. In a calm sky, the positive (+) and negative (-) charges are evenly spaced throughout the atmosphere. Therefore, a calm sky has a neutral charge.
Inside a thunderstorm, the electric charge is spread out differently. A thunderstorm is made up of ice crystals and hailstones. The ice crystals have a positive charge, and the hailstones have a negative charge. An updraft pushes the ice crystals to the top of the thunderstorm cloud. At the same time, the hailstones are pushed to the bottom of the thunderstorm by its downdraft. These processes separate the positive and negative charges of the cloud into two levels: the positive charge at the top and the negative charge at the bottom.

During a thunderstorm, the Earth's surface has a positive charge. Because opposites attract, the negative charge at the bottom of the thunder cloud wants to link up with the positive charge of the Earth's surface.
Once the negative charge at the bottom of the cloud gets large enough, a flow of negative charge rushes toward the Earth. This is known as a stepped leader. The positive charges of the Earth are attracted to this stepped leader, so a flow of positive charge moves into the air. When the stepped leader and the positive charge from the earth meet, a strong electric current carries positive charge up into thecloud. This electric current is known as the return stroke and humans can see it as lightning.
TYPES OF LIGHTNING:
Lightning can take place in several different areas of a thunderstorm. Most lightning (about 80%) occurs within a single cloud and is called cloud-to-cloud lightning. Most of the other 20% of lightning involves a stroke from the cloud to the ground. Damage is usually caused where the lightning strikes the ground. And sometimes lightning can jump from one cloud to another or to the surrounding air.
Most of the lightning we see appears as a single line of bright white light, called streak lightning. However, several other types of lightning can occur.
Forked lightning occurs when a second lightning stroke doesn't follow the same path as the first lightning stroke. It usually follows a zigzag pattern and appears forked with many branches. Forked lightning can go from cloud-to-ground, cloud-to-cloud, or cloud-to-air.
Ribbon lightning occurs in thunderstorms with high cross winds and multiple strokes of lightning. Winds separate the strokes of the lightning bold, making it look like there are parallel streaks of light. This is a form of cloud-to-ground lightning.
Bead lightning is a relatively rare form of lightning. In bead lightning, the stroke appears to break up into a string of short, bright sections, and looks like a string of beads. This is a form of cloud-to-ground lightning.
Sheet lightning occurs when the actual bolt or flash of lightning is hidden behind the clouds. When sheet lightning occurs the entire sky flashes a glowing white color. This is a form of cloud-to-cloud lightning.
Heat lightning occurs within a cloud, but the observer is too far away from the storm for its thunder to be heard. The sound waves dissipate before reaching the observer. Instead of individual strokes, heat lightning often lights up the entire cloud. This is a form of cloud-to-cloud lightning.










