
Introduction to Energy Level Formula:
Definition of Energy Levels: Energy levels refer to the quantized states of energy that electrons in atoms, molecules, or solid materials can occupy. These energy levels determine the electronic structure and behavior of materials, particularly in the context of electrical conductivity. Significance of Energy Levels: Energy levels are essential in understanding the electrical properties of conductors and semiconductors. They dictate how easily electrons can move through a material, which is crucial for various electronic applications.Energy Levels in Conductors:
Band Theory of Solids: The Band Theory of Solids describes the arrangement of energy levels in a solid material. In conductors, the energy bands are partially filled, allowing electrons to move freely, contributing to high electrical conductivity. Valence Band and Conduction Band: The valence band is the highest energy band occupied by electrons at absolute zero temperature, while the conduction band is the next higher energy band that is empty or partially filled. The energy gap between them determines the conductivity of the material. Fermi Level: The Fermi level represents the highest energy level at which electrons are present at absolute zero. It plays a crucial role in determining the electrical behavior of conductors.Also Check - Energy Formula
Energy Band Diagram:
Energy band diagrams provide a graphical representation of the energy levels in a conductor. They help visualize the energy gap between the valence and conduction bands. Electrical Conductivity in Conductors: Conductors have high electrical conductivity due to the presence of free electrons in the conduction band. This conductivity can be described using mathematical formulas such as Ohm's Law and the Drude model.Also Check - Optics Formula
Mathematical Formulas:
- Ohm's Law: I = V/R
- Drude Model for Electrical Conductivity:
Also Check - Ray Optics Formula
Energy Levels in Semiconductors:
Intrinsic and Extrinsic Semiconductors: Semiconductors can be classified as intrinsic (pure) or extrinsic (doped) based on their electron concentration. Intrinsic semiconductors have a limited number of free electrons and holes, while extrinsic semiconductors are intentionally doped to increase their conductivity. Energy Band Diagram for Semiconductors: Semiconductor energy band diagrams illustrate the energy levels in these materials, including the valence band, conduction band, and the position of the Fermi level. The energy gap in semiconductors is smaller than in insulators but larger than in conductors. Fermi Level in Semiconductors: The Fermi level in semiconductors lies between the valence and conduction bands, and its position determines whether a semiconductor behaves as an n-type or p-type material. Carrier Generation and Recombination: Semiconductors can generate and recombine electron-hole pairs when exposed to light or an electric field, making them essential in optoelectronic applications.Also Check - Elasticity Formula
Temperature Effects:
The position of the Fermi level in semiconductors is temperature-dependent. As temperature increases, the number of carriers also increases, affecting the electrical behavior. Mathematical Formulas:- Intrinsic Carrier Concentration (n_i):
- Relationship between Electron and Hole Concentrations in Intrinsic Semiconductors:
- Carrier Concentration in Extrinsic Semiconductors (n-type and p-type):
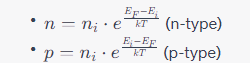 - Where:
- E
_F
is the Fermi level energy.
- E
_i
is the intrinsic energy level.
- Where:
- E
_F
is the Fermi level energy.
- E
_i
is the intrinsic energy level.
Applications of Energy Levels in Conductors and Semiconductors:
Electronics and Semiconductor Devices: Conductors and semiconductors are fundamental to the operation of electronic devices such as transistors, diodes, and integrated circuits, which are the building blocks of modern electronics.Conductors in Electrical Circuits:
Conductors are used to transmit electrical energy efficiently in power distribution systems, electrical wiring, and electronic circuits.Optoelectronic Devices:
Semiconductors are the basis of optoelectronic devices like light-emitting diodes (LEDs), photodetectors, and solar cells.Energy Harvesting:
Semiconductor materials are employed in energy harvesting systems, converting light or heat energy into electrical power Understanding energy levels in conductors and semiconductors is crucial for designing and developing electronic devices and systems. The energy band theory and related mathematical formulas provide the foundation for exploring and harnessing the electrical properties of these materials. As technology continues to advance, a deep understanding of energy levels will remain essential for innovation in the field of electronics and materials science.Energy levels FAQs
What are energy levels in materials, and why are they important?
Energy levels represent quantized states of energy where electrons can exist. They are essential because they determine how easily materials conduct electricity. In conductors, energy levels enable the flow of electrons, while in semiconductors, they influence electronic behavior and device functionality.
How do energy bands differ in conductors, semiconductors, and insulators?
In conductors, the valence and conduction bands overlap, allowing free electron movement. Semiconductors have a small energy gap between these bands, while insulators have a large energy gap where electrons cannot move freely.
What is the Fermi level, and why is it significant in materials science?
The Fermi level is the highest energy level at absolute zero temperature where electrons are present. It is crucial because it determines a material's electrical properties. In semiconductors, its position relative to the energy bands defines whether the material is n-type or p-type.
How does temperature affect the electrical behavior of semiconductors?
Temperature affects the number of charge carriers (electrons and holes) in semiconductors. As temperature increases, more electron-hole pairs are generated, impacting conductivity. The position of the Fermi level also changes with temperature.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.
Get App









