
Concentration
Chemical Reaction And Equation of Class 10
Solution is a homogenous mixture of two or more components. A solution consists of a dissolved substance known as solute and a substance in which the solute is dissolved is known as solvent. The concentration of a solution reflects the relative proportion of solute and solvent present in the solution. The various concentration terms are
1. % w/W (Weight percent or mass percent)
% w/ W =

10% H 2 SO 4 w/w means 10 grams of pure H 2 SO 4 is present in 100 gram of H 2 SO 4 solution.
2. % w/V (Weight/volume)

10% w/V H 2 SO 4 solution means 10 gram of pure H 2 SO 4 is present in 100 ml of H 2 SO 4 solution.
3. % v/V (volume/volume)
This concentration term is defined for a solution of liquid into liquid
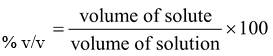
42.8% v/V ethanol solution means that 42.8 ml pure ethanol is present in 100 ml of ethanol solution.
4. Molality (m): It is defined as no. of moles of solute present in 1 kilogram of solvent.
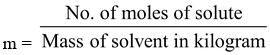
5.
Mole Fraction
(X): Suppose a solution of A and B contains nA moles of A and nB moles of B then mole fraction of A(XA) =
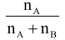
Similarly mole fraction of B(XB) =
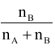
It is clear that
XA + XB = 1.
Out of all the concentration terms we will discuss molarity and normality in detail because these two concentration terms are related with two important methods of stoichiometry i.e.
(i) Method of Moles
(ii) Method of Gram equivalents
6. Molarity (M): It is defined as the number of moles of solute dissolved in one litre of solution
M = No. of Moles of solute/Volume of solution in litre
Suppose w gram solute is dissolved in Vml solution and molecular weight of solute
is m.
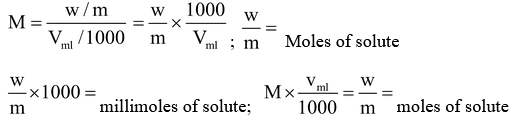
So if a substance is in solid form then it’s moles or millimoles can be calculated by using w/m or w/m x 100 and when the same substance is in solution then M x V l or M x V ml will give the mole or mill moles respectively.
7. Parts per million (ppm) : It is defined as the quantity of the solute in grams present in 106 grams of the solution

Atmospheric pollution in cities is also expressed in ppm by volume. It refers to the volume of the pollutant in 106 units of volume. 10 ppm of SO2 in air means 10 mL of SO2 in present in 106 mL of air.
Illustration 5 : Calculate the total number of oxalic acid molecules present in a 100 ml of 0.01 M oxalic acid solution.
Solution : No. of millimoles of solute (oxalic acid) present = M x V ml = 0.01 x 100 = 1.
Since 1 mole of any substance contains 6.023 x 10 23 molecules so 1 millimole will contain 6.023 x 10 20 molecules.
Illustration 6 : A 2 M aqueous solution of Potash Alum [K2SO4. Al2 (SO4)3 24H2O], an ionic solid is prepared. Calculate moles of SO² 4 ¯ present in 800 ml of solution.
Solution : Moles of solute (Potash Alum) present in 800 ml of solution
=
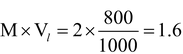 .
.
Since potash alum is an ionic solid. So it will split into ions in solution.

it is apparent from balanced equation than 4 moles of SO² 4 ¯are produced from 1 mole of potash alum.
So moles of SO² 4 ¯present = 1.6 x 4 = 6.4.










