
Transition Elements
Coordination Compound of Class 12
In periodic table, the d−block elements are placed between s−block and p−block elements. These are called transition elements because they show a gradual gradation in periodic properties. The transition elements are mainly those elements which either in atomic or ionic state contain partially filled d−subshells. It must be noted that the elements of group 12 (Zn, Cd, and Hg) have completely filled d−subshells in atomic as well as in ionic form, so they do not truly represent transition elements. Moreover, they also do not exhibit general properties of transition elements like variable oxidation states, coloured compounds, formation of complex compounds etc. However, they are studied with transition elements just to maintain the status of the periodic table. Further, it must be noted that the elements of group 3 (Sc, Y, La and Ac) differ in their properties from those of other transition elements (they are uniformly trivalent, diamagnetic and colourless).
Electronic Configuration
Transition elements involve the filling of d−subshell of penultimate shell. The general electronic configuration is
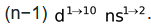 On the basis of the principal quantum number of partially filled d−subshell, d−block elements are classified into four series (3d, 4d, 5d and 6d) series, of which 6d series is still incomplete whereas 3d, 4d and 5d series contain 10 elements each and are complete.
On the basis of the principal quantum number of partially filled d−subshell, d−block elements are classified into four series (3d, 4d, 5d and 6d) series, of which 6d series is still incomplete whereas 3d, 4d and 5d series contain 10 elements each and are complete.
General Characteristics of d−Block Elements
Metallic Character: All the transition elements are metallic in nature. They have tendency to form strong covalent bonds due to partially filled d−subshell (except Zn, Cd, Hg) as well as strong metallic bond due to large number of electrons that can be lost. So their melting and boiling points are very high (more than 1000°C except Zn, Cd, and Hg), they have high densities and are very hard and brittle.
Atomic Radii: Due to small screening effect of d−subshell the atomic radius decreases with increase in atomic number. The size of transition elements is small due to which their densities and ionisation energies are high.
Oxidation States: During formation of compounds, d−orbitals of inner shell can also participate in bonding. Hence the transition elements exhibit variable oxidation states (except 3rd group and 12th group elements). The oxidation state of 3rd group elements (Sc, Y, La, Ac) is +3 whereas the oxidation state of 12th group (Zn, Cd, Hg) is +2 only. In lower oxidation states (+2, +3), the bonds formed are ionic, whereas, in higher oxidation states the bonds formed are covalent. The highest possible oxidation state is +8 for transition elements.
Ionisation Energy: Ionisation energy of transition elements is in between those of
s−block and p−block elements. Ionisation energy of transition elements in a particular series increases due to increase in nuclear charge. It must be kept in mind that the ionisation energies of 5d−elements is more than 3d and 4d−elements because of very poor shielding of the nucleus by 4f−electrons.
Complex formation: Transition metal ions have a great tendency to form complexes with other molecules or ions (which can donate a pair of electron to form a coordinate bond) called ligands. This property of formation of complexes is due to vacant d−orbitals (which can accept electron pair) and high nuclear charge with small size which facilitates the acceptance of electrons from the ligands.
The complexes of transition elements of small size are more stable as compared to those of large size elements in a particular series with same oxidation state. If the oxidation state is different for the same metal, then the complex with greater oxidation state of central metal will be more stable.
Colour: The d−orbitals of the transition metal ions and their complexes do not have same energies. Under the influence of anions or ligands, the d−orbitals split into two sets. The energy difference of these two sets corresponds to visible region of spectrum. Due to the absorption of visible radiation for d−d transitions, most of the compounds and complexes of transition elements are coloured. The colour will appear if the central metal contains partially filled d−subshell. But if the d−subshell is completely filled, the complex shall be colourless (eg. Cu+, Ag+, Sc3+ etc). The colour of the complex ion is attributed to the presence of unpaired electrons in d−subshells which undergoes d−d transition.
Colour due to Charge Transfer: When the compounds absorb light, electrons can move from reducing agent to oxidising agent. Due to the movement of the electrons, colour is shown by the compounds. This is called charge transfer.
Now, charge transfer can occur in two ways
(i) Ligand to Metal Charge Transfer: In KMnO 4 and K 2 Cr 2 O 7 , both strongly coloured, Mn and Cr ions do not have any electrons in the d−orbitals. In KMnO4, oxidation number of Mn is +7, i.e. it has lost all seven electrons present in 3d 5 and 4s 2 orbitals. Similar is the case with Cr in K 2 Cr 2 O 7 , it has lost all the six electrons from 3d 5 and 4s 2 orbitals. As there are no electrons in the 3d−orbitals, d−d transition cannot occur. But now ligand [oxygen] has acquired lot of negative charge (electrons) and electrons can jump from high density oxygen atoms (ligands) to the vacant Mn/Cr d−orbitals. They absorb sunlight and jump.
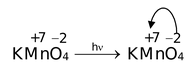
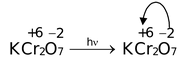
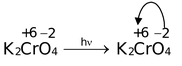
Other examples are,
(a)
(b)
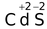
Electronic configuration of Cd is
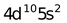
Electronic configuration of Cd 2+ is 4d 10 (Full−filled d−orbital)

(c) P +2 b -2 s
Electronic configuration of PbS is 6s 2 6p 2
Electronic configuration of Pb 2+ is 6s 2 .
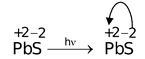
(ii) Metal to Metal Charge Transfer: Consider the following reactions
(a)
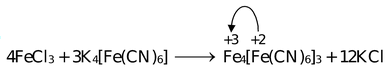
(b)
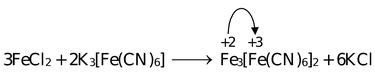
Treatment of Fe(III) solution with K4[Fe(CN)
6
] forms a deep blue precipitate (Prussian Blue) Fe
4
[Fe(CN)6]3 and Fe(II) solution when treated with K
3
[Fe(CN)
6
] forms again a deep blue precipitate (Turnbull's blue) Fe
3
[Fe(CN)
6
]
2
. The blue colour is due to charge transfer from
 and
and
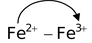 in the reaction (a) and (b) respectively.
in the reaction (a) and (b) respectively.
Catalytic Properties: Transition metals and their compounds can adsorb large number of substances on their surface, which reacts faster due to increased concentrations at surface (adsorption theory of catalysis). So they can catalyse many reactions.
Transition metals can form intermediate compounds with many substances due to variable oxidation states. So they provide new paths of lesser activation energies for several chemical reactions (activated complex theory). And so the rates of reactions will be increased by using these compounds as catalyst.
Magnetic Properties: Substances can be classified into two categories on the basis of magnetic behaviour. Some substances are slightly repelled by magnetic field, so they weigh less in magnetic field and are called diamagnetic substances. Second category of substances are attracted by magnetic field, so they weigh more in magnetic field and are called paramagnetic substances.
If all the electrons of the transition metal atom or its ion in a compound are paired, the substance will be diamagnetic. And if any unpaired electron is present, the compound will be paramagnetic. Further, paramagnetism increases with increase in number of unpaired electrons and is given as
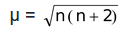 B.M. (Bohr Magneton)
B.M. (Bohr Magneton)
where n is the number of unpaired electrons and B.M. is the unit of magnetic moment.
Reducing Character: The reduction potentials of transition elements (except Cu & Hg) are negative as compared to that of hydrogen electrodes, so they can liberate H2 gas on treatment with acids. But sometimes transition elements other than Cu & Hg also do not displace hydrogen from acidic solutions due to the formation of a layer of inert oxides at their surface.
Formation of Alloys: One transition metal can replace another metal in crystal lattice due to similar size, resulting in the formation of alloys or solid solutions. These alloys have generally high melting points and are hard.
Interstitial Compounds: Transition metals can take up atoms of small size (H, C, N) in the voids of their lattices to form interstitial compounds which have more tensile strength.
Lanthanides and Actinides
The f−block elements consist of two series of inner transition elements i.e. lanthanides and actinides. They are also called rare earth elements. Lanthanides involve the filling of 4f−orbitals while actinides involve the filling of 5f−orbitals in their atoms. These f−orbitals belong to the shell, which is third from the outer shell. This factor accounts for some characteristic properties of these elements.
(A) Lanthanides
The series of elements involving the filling of 4f−orbitals is called lanthanide series. Lanthanum, the first member of lanthanides has the configuration of 5d 1 6s 2 and next member Cerium has 4f 2 6s 2 , while the next element Praseodymium has the configuration . 4f 3 6s 2 , Although Lanthanum itself does not possess any 4f electrons, it is customary to include this element in the series. The electronic configuration of the elements with fully filled (f 14 ) and half−filled (f 7 ) f−orbitals is relatively more stable. The extra stability of half−filled orbitals is seen in the elements Europium ( 4f 7 5d 0 6s 2 ,) and Gadolinium ( 4f 7 5d 1 6s 2 ). The element Ytterbium has fully−filled f−orbitals with the configuration 4f 14 5d 0 6s 2 and the extra electron in Lutetium goes in 5d−orbitals ( 4f 14 5d 1 6s 2 ). So except for Lanthanum, Gadolinium and Lutetium, which have a single electron in 5d−orbitals, the lanthanides do not have electrons in the 5d−orbitals.
Physical and Chemical Properties of lanthanides
Lanthanides are silvery white metals having low tensile strengths. They are good conductors of heat and electricity. Some of their physical properties are
(a) Density : Lanthanides have high densities ranging between 6.77 to 9.74 g cm -3 . The densities, in general, increase with increase in atomic number.
(b) Melting and boiling points: Lanthanides have fairly high melting points. However, no definite trend is observed in melting and boiling points from La to Lu.
(c) Ionisation energies: Lanthanides have farily low ionization energies. The IE1 and IE2 values are quite comparable to those of alkaline earth metals, particularly Calcium.
(d) Electropositive character: Lanthanides have high electropositive character due to low ionization energies.
(e) Coloured ions: Many of the lanthanide ions are coloured in solid state as well as in solutions. The colour is attributed to f−f transitions since they have partly filled f−orbitals.
(f) Magnetic behaviour: The lanthanide ions (M +3 ) generally show paramagnetism due to the presence of unpaired electrons in f−orbitals.
(g) Oxidation States: The lanthanides exhibit a principal oxidation state of +3. The +3 oxidation states in Lanthanum, Gadolinium and Lutetium are especially stable because +3 ions of these elements have an empty, a half−filled and completely filled 4f−subshell respectively. Cerium and Terbium also exhibit oxidation state of +4. Ce 4+ has configuration 4f 0 and Tb +4 has the configuration 4f 7 . Sm 2+ (4f 6 ), Eu 2+ (4f 7 ) and Yb 2+ (4 f 14 ) ions also exist in aqueous solutions. Although a few lanthanides exhibit oxidation states of +4 and +2, yet they have the tendency to attain the oxidation state of +3 because the +3 oxidation state is most stable state for all lanthanides. For example,Ce 4+ is a good oxidizing agent while Sm 2+ is a good reducing agent.
Ce 4+ + Fe 2+ → Ce 3+ + Fe +
2Sm 2+ + 2H2O → 2Sm 3+ + 2OH− + H 2
In the above reactions, Ce 4+ and Sm 2+ are converted into Ce 3+ and Sm 3+ respectively.
(h) Chemical reactivity: Many lanthanides react with carbon to form salt−like carbides and with hydrogen to give salt−like hydrides. Lanthanides react with oxygen and sulphur to form oxides (M 2 O 3 ) and sulphides (M 2 S 3 ) respectively. Cerium gives CeO 2 . The M 2 O 3 types of oxides react with water to form insoluble hydroxides. Oxides and hydroxides on reaction with CO 2 give carbonates, M2(CO 3 ) 3 . Lanthanide compounds are generally ionic. These compounds are generally coloured and exhibit paramagnetism. These elements occur together in nature because of their great chemical similarity and are difficult to separate. However, because of lanthanide contraction they do exhibit slight variation in their properties and this becomes the basis of their separation by ion−exchange method.
(i) Atomic/ionic radii and Lanthanide contraction: The atomic and ionic radii of tri−positive lanthanide ions(M 3+ ) show a steady and gradual decrease in moving from La to Lu. Although the atomic radii do show some irregularities but ionic radii decrease steadily from La to Lu. The steady decrease in the size of lanthanide ions (M 3+ ) with the increase in atomic number is called lanthanide contraction.
Cause of lanthanide contraction: As the atomic number increases in lathanide series, for the addition of every proton in the nucleus, the extra electron goes to fill 4f−orbitals. The 4f−electrons constitute inner shells and are rather ineffective in screening the nuclear charge. Thus, there is a gradual increase in the effective nuclear charge experienced by the outer electrons. Consequently, the attraction of the nucleus for the electrons in the outermost shell increases as the atomic number of lanthanides increases and the electron cloud shrinks. This results in gradual decrease in the size of lanthanides with increase in atomic number.
Consequences of Lanthanide Contraction:
(i) Similarity of second and third transition series : The atomic radii of second row of transition elements are almost similar to those of the third row of transition elements. For example, among the elements of group 3, there is normal increase in size from Sc to Y to La. But after lanthanides the atomic radii from second to third transition series do not increase for groups 4 and 5 etc.
Here the usual increase in size on moving down the group from second to third transition elements is cancelled by the decrease in size due to lanthanide contraction. Also as a result of lanthanide contraction, the second and third rows of transition elements resemble each other more closely than do the first and second row.
(ii) Separation of lanthanides: Separation of lanthanides is also possible due to lanthanide contraction. All the lanthanides have quite similar properties and due to this reason they are difficult to be separated. However, because of lanthanide contraction their properties (such as ability to form complexes) vary slightly. This slight variation in properties is utilized in the separation of lanthanides by ion exchange method.
(iii)Variation in basic strength of hydroxides: The basic strength of hydroxides decreases from La(OH) 3 to Lu(OH) 3 . Due to lanthanide contraction, size of M 3+ ions decreases and there is increase in the covalent character in M−OH bond.
(B) Actinides
The series of elements involving the filling of 5f−orbitals is called actinide series. They follow Ac (89) and include elements from Th(90) to Lw(10 3 ).
The chemistry of actinides is more complicated due to the existence of greater range of oxidation states for these metals. Moreover, all these metals are radioactive and therefore, their accessibility for laboratory investigations is limited. The elements beyond uranium are all man−made elements and are made by nuclear−chemical methods. Some of the physico−chemical properties of actinides are discussed as follows.
Some Physio−chemical properties of Actinides
(a) Oxidation states: The common oxidation state of these elements is +3. However, they also exhibit oxidation states of +4, +5, +6.
(b) Physical appearance : Actinides are silvery white metals. They get tarnished when exposed to alkalies.
(c) Density: All the actinides except Thorium and Americum have high densities.
(d) Colour: The actinide ions in general are coloured. The appearance of colour depends upon the number of 5f−electrons. The ions containing 5f 0 and 5f 7 configurations are colourless, while those having 2 to 6 electrons in 5f−shell are coloured.
U 3+ (5f 3 ): Red, Np 3+ (5f 4 ): Bluish, Pu 3+ (5f 5 ): Blue, Am 3+ (5f 5 ): Pink
(e) Ionisation energies: Ionization energy values of actinides are low.
(f) Electropositive character: All the known actinide metals are highly electropositive. They resemble lanthanide series in this respect.
(g) Melting and boiling points: They have high melting and boiling points. They do not follow regular gradation of melting or boiling point with increase in atomic number.
(h) Magnetic properties: The actinide elements are paramagnetic due to the presence of unpaired 5f−electrons.
(i) Radioactive nature:
All the actinides are radioactive in nature.
(j) Actinide contraction:
The size of atom/cation decreases regularly along the actinide series. The steady decrease in ionic radii with increase in atomic number is referred to as actinide contraction. This is due to poor shielding of 5f−electrons.









