
Isomerism In Co-ordination Compounds
Coordination Compound of Class 12
The compounds having same molecular formula but different physical and chemical properties on account of different structures are called isomers and the phenomenon as isomerism. Isomerism in coordination compounds may be divided into two main types:
(1) Structural isomerism, (2) Stereo−isomerism
Structural Isomerism
It is displayed by compounds that have different ligands within their coordination spheres. The different types of structural isomerism shown by coordination compounds are
(i) Ionization isomerism: This type of isomerism arises when the coordination compound give different ions in solution. For example, there are two isomers of the formula Co(NH3)5BrSO4.
[Co(NH 3 ) 5 Br]SO 4 [Co(NH 3 ) 5 Br] 2 + +
(Violet) Pentaamminebromo−
cobalt(III) ion
This isomer gives a white precipitate of BaSO 4 with BaCl 2 solution.
[Co(NH 3 ) 5 SO 4 ]Br [Co(NH 3 ) 5 SO 4 ] + +Br−
(Red) Pentaamminesulphato−
cobalt(III) ion
Other examples of ionization isomerism are:
[Pt(NH 3 ) 4 Cl 2 ]Br 2 and [Pt(NH 3 )4Br 2 ]Cl 2
Tetraamminedichloroplatinum(IV) Tetraamminedibromoplatinum(IV)
bromide chloride
[Co(NH 3 )4Cl 2 ]NO 2 and [Co(NH 3 ) 4 ClNO 2 ]Cl
Tetraamminedichlorocobalt(III) Tetraamminechloronitrocobalt(III)
nitrite chloride
(ii) Hydrate isomerism: This type of isomerism arises when different number of water molecules are present inside and outside the coordination sphere. This isomerism is best illustrated by the three isomers that have the formula CrCl 3 .6H 2 O.
(a) [Cr(H 2 O) 6 ]Cl 3 : Violet. All the six water molecules are coordinated to Cr. It has three ionisable chloride ions.
(b) [Cr(H 2 O) 5 Cl]Cl 2 .H 2 O: Green. Five water molecules are coordinated to Cr. It has two ionisable chloride ions. One water molecule outside the coordination sphere can be easily lost.
(c) [Cr(H 2 O) 4 Cl 2 ]Cl. 2 H 2 O: Green. Four water molecules are coordinated to Cr. It has one ionisable Cl− ion. Other examples of hydrate isomerism are:
[Co(NH 3 )4(H 2 O)Cl]Br 2 and [Co(NH 3 ) 4 (Br) 2 ]Cl.H 2 O
Tetraammineaquachlorocobalt(III) Tetraamminedibromocobalt(III)
bromide chloride 1−water
[Cr(en) 2 (H 2 O)Cl]Cl 2 and [Cr(en) 2 Cl 2 ]Cl.H 2 O
Aquachlorobis(ethylenediamine) Dichlorobis(ethylenediamine)
chromium(III) chloride chromium(III) chloride 1−water
(iii) Coordination isomerism: This type of isomerism is observed in the coordination compounds having both cationic and anionic complex ions. The ligands are interchanged in both the cationic and anionic ions to form isomers. Some examples are:
[Pt(NH 3 ) 4 ][CuCl 4 ] and [Cu(NH 3 ) 4 ] [PtCl 4 ]
Tetraammineplatinum(II) Tetraamminecopper(II)
tetrachlorocuprate(II) tetrachloroplatinate(II)
[Cr(NH 3 )6] [Co(C 2 O 4 ) 3 ] and [Co(NH 3 ) 6 ] [Cr(C 2 O 4 ) 3 ]
Hexaamminechromium(III) Hexaamminecobalt(III)
trioxalatocobaltate(III) trioxalatochromate(III)
[Cr(NH 3 ) 6 ] [Cr(SCN) 6 ] and [Cr(NH 3 ) 4 (SCN) 2 ] [Cr(NH 3 ) 2 (SCN) 4 ]
Hexaamminechromium(III) Tetraamminedithiocyanatochromium(III)
hexathiocyanatochromate(III) diamminetetrathiocyanatochromate(III)
(iv) Linkage isomerism: This type of isomerism occurs in complex compounds which contain ambidentate ligands like , SCN−, CN−, and CO. These ligands have two donor atoms but at a time only one atom is directly linked to the central metal atom of the complex. These type of isomers are distinguished by infra−red (I.R.) spectroscopy. For example [Co(NH 3 ) 5 NO 2 ]Cl2 and [Co(NH 3 ) 5 ONO]Cl 2 are linkage isomers as is linked through N or through O.
[Co(NH 3 ) 5 NO 2 ]Cl 2 and [Co(NH 3 ) 5 ONO]Cl 2
Pentaamminenitrocobalt(III) Pentaamminenitritocobalt(III)
chloride (yellow) chloride (red)
[Pd(dipy)(SCN) 2 ] and [Pd(dipy)(NCS) 2 ]
Dipyridyldithiocyanato Dipyridyldiisothiocyanato
palladium(II) palladium(II)
(v) Polymerisation isomerism: This type of isomerism exists in compounds having same stoichiometric composition but different molecular compositions. The molecular compositions are simple multiples of the simplest stoichiometric arrangement. For example in the following three compounds, the second and third compounds are polymers of the first.
[Pt(NH 3 ) 2 Cl 2 ] [Pt(NH 3 ) 4 ] [PtCl 4 ] [Pt(NH 3 ) 3 Cl] 2 [PtCl 4 ]
(i) (ii) (iii)
[Note: (ii) and (iii) compounds are actually not the examples of polymerization, i.e. (i) compound is not acting as a monomer in the formation of (ii) and (iii) compounds.]
(vi)Coordination position isomerism: This type of isomerism is exhibited by polynuclear complex by changing the position of ligands with respect to different metal atoms present in the complex. For example,
and Unsymmetrical Symmetrical (Both the chloro ligands are with (Same ligands are linked with both same cobalt ion) cobalt ions)
Stereo−Isomerism
Compounds are stereo−isomers when they contain the same ligands in their coordination spheres but differ in the way that these ligands are arranged in space. Stereo−isomerism is of two types, viz., geometrical isomerism and optical isomerism.
(I) Geometrical isomerism: This isomerism is due to ligands occupying different positions around the central metal atom or ion. The ligands occupy positions either adjacent or opposite to one another. This type of isomerism is also known as cis−trans isomerism.
Geometrical isomerism is very much common in coordination number 4 and 6 isomerism.
Square planar complexes (coordination number four) exhibit geometrical isomerism.
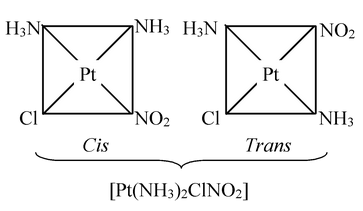
(i) Complexes with general formula, Ma 2 b 2 (where both a and b are monodentate) can have cis− and trans−isomers.
(ii) Complexes with general formula Ma 2 bc can have cis− and trans−isomers
(iii) Complexes with general formula, Mabcd, can have three isomers
Example: [Pt(NH 3 )(NH 2 OH)(NO 2 )(py)]NO 2 .
(iv) Square planar complexes having unsymmetrical bidentate ligands can also show geometrical isomerism. For example, platinum glycinato complex, [Pt(Gly) 2 ], exhibits geometrical isomerism.
Cis−isomer Trans−isomer
Octahedral complexes of the type Ma4b2 and Ma 3 b 3 exhibit geometrical isomerism.
Example: [Co(NH 3 )4Cl 2 ]Cl [Co(NH 3 )3Cl 3 ]
Octahedral complexes of general formula, Mabcdef, can have fifteen geometrical isomers.
Note:
(i) Geometrical isomerism is not observed in complexes of coordination number 2 and 3.
(ii) Geometrical isomerism is not observed in complexes of coordination number 4 of tetrahedral geometry.
(iii) The complexes of general formulae, Ma3b or Mab3 , or Ma4 of square planar geometry do not show geometrical isomerism.
(iv) The complexes of general formula, Ma6 and Ma5b of octahedral geometry do not show geometrical isomerism.







