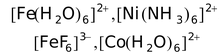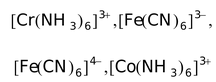VBT For Bonding In CO−Ordination compounds
Coordination Compound of Class 12
This theory was proposed by Linus Pauling. According to this theory, the structures of the complexes can be explained on the basis of hybridization. Orbitals of central metal atom/ion is hybridized and each coordinating groups (i.e. ligands) give a pair of electrons. These pair of electrons are accommodated in the vacant hybrid orbitals of the metal atom/ion. For example, combination of one s− and three p−orbitals of the valence shell gives four hybrid orbitals of equivalent energy. This is known as Sp 3 −hybridization. Besides this, in complexes, dSp 2 (square planar), d 2 Sp 3 and Sp 3 d 2 (octahedral) hybridizations are also very common.
The important features of Valence Bond Theory are as follows
(a) In this approach, the basic assumption made is that the metal−ligand bond arises by the donation of pair of electrons by ligands to the metal atom/ion.
(b) In order to accommodate these electrons, the metal atom/ion must possess requisite number of vacant orbitals of comparable energy. These orbitals of metal atom/ion undergo hybridization to give a set of hybrid orbitals of equal energy.
(c) In complex formation, Hund's rule of maximum multiplicity is strictly followed. However, in complex formation redistribution of electrons and complete pairing of electrons may take place. In this way, some orbitals are vacated and made available for hybridization.
A strong ligand donates a pair of electrons easily whereas a weak ligand with difficulty. Under the influence of strong ligands, the metal electrons are forced to pair up contrary to Hund's rule.
Sometimes, the unpaired (n−1) d−electrons pair up as per the need prior to bond formation thus making some (n−1) d−orbitals vacant. The central metal atom then makes available a number of empty orbitals (equal to its coordination number) for the formation of coordinate bonds with suitable ligand orbitals.
(d) With the approach of the ligands, metal−ligand bonds are formed by the overlap of these hybrid orbitals with those of the ligands i.e. by donation of electron pair by the ligands to the empty hybridized orbitals. Consequently these bonds are of equal strength and directional in nature.
(e) A substance which do not contain any unpaired electron is not attracted by a magnet. It is said to be diamagnetic. On the other hand, a substance which contains one or more unpaired electrons in the d−orbitals, is attracted by a magnetic field [exception O2 and NO]. It is said to be paramagnetic.
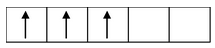
Paramagnetism can be calculated by the expression,
µs =
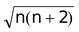 , where µ = magnetic moment (which is calculated in Bohr magneton
, where µ = magnetic moment (which is calculated in Bohr magneton
i.e. B.M.),
s = spin only value and n = number of unpaired electrons.
Hence,
if n = 1, µs =
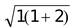 = 1.73 B.M.
= 1.73 B.M.
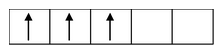
if n = 2; µs =
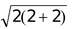 = 2.83 B.M.
= 2.83 B.M.
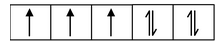
if n = 3; µs =
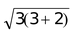 = 3.87 B.M. and so on
= 3.87 B.M. and so on
On the basis of value of magnetic moment, we can predict the number of unpaired electrons present in the complex. If we know the number of unpaired electrons in the metal complex, then it is possible to predict the geometry of the complex species.
(f) Octahedral, square planar and tetrahedral complexes are formed as a result of d 2 Sp 3 (or Sp 3 d 2 ), dSp 2 and Sp 3 hybridisation respectively.
For predicting the type of structure (geometry) of a complex species, the following guidelines would be helpful.
(i) Electronic configuration of first transition series
|
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
|
3d 1 4s 2 |
3d 2 4s 2 |
3d 3 4s 2 |
3d 5 4s 1 |
3d 5 4s 2 |
3d 6 4s 2 |
3d 7 4s 2 |
3d 8 4s 2 |
3d 10 4s 1 |
3d 10 4s 2 |
(ii) The complexes having co−ordination number 4 and 6 are in IIT−JEE syllabus. For co−ordination number 4, the hybridizations possible are
Sp
3
and
dSp
2
, having tetrahedral and square planar geometries respectively, while for co−ordination
number 6, the hybridizations possible are
d
2
Sp
3
and
Sp
3
d
2
, having octahedral geometry in both the cases.
(iii) A complex would be paramagnetic, if it has at least one unpaired electron and would be diamagnetic if no unpaired electron is present.
(iv) There are two types of ligands namely strong field and weak field ligands. A strong field ligand is capable of forcing the electrons of the metal atom/ion to pair up (if required). Pairing is done only to the extent which is required to cause the hybridization possible for that co−ordination number. A weak field ligand is incapable of making the electrons of the metal atom/ion to pair up.
Strong field ligands: CN−, CO, en, NH 3 , H 2 O, NO 2 - , Py
Weak field ligands: I−, Br−, Cl−, F−, NO 3 - , OH−, C 2 O 4 2- , NH 3 , H 2 O
In most of the complexes, NH 3 acts as a strong ligand and H 2 O acts as a weak ligand. But, in few cases, NH3 behaves as a weak ligand whereas H 2 O as a strong ligand because it is on border line. By knowing the value of magnetic moment, it may be suggested that whether they act as a weak ligand or a strong ligand in a particular complex.
(v) The complex species may or may not be coloured, if the unpaired electrons are present in it while it would be colourless if no unpaired electrons are present.
(vi) The VBT explains the bonding and geometry of only those complexes which have only one type of ligands, either strong field or weak field ligands.
(vii)The common types of hybridizations are
|
C.N. |
Hybridization |
Geometry |
Example |
|
2 |
sp |
Linear |
[Ag(NH 3 ) 2 ]Cl, K[Ag(CN) 2 ] |
|
3 |
sp 2 |
Trigonal planar |
K[HgI 3 ] |
|
4 |
sp 3 |
Tetrahedral |
where X = Cl−, Br−, I− |
|
dsp 2 |
Square planar |
|
|
|
5 |
sp 3 d |
Square pyramidal |
[SbF 5 ] 2- |
|
dsp 3 |
Trigonal bipyramidal |
Fe(CO) 5 , [CuCl 5 ] 3- |
|
|
6 |
Sp 3 d 2 , |
Octahedral |
|
|
d 2 Sp 3 , |
Octahedral |
|
Now, let us apply these assumptions of valence bond theory to the formation of following types of complexes.
Four Co−ordinated Complexes
The complexes in which the co−ordination number of metal atom or ion is 4, is known as
four co−ordinated complexes. For the formation of four co−ordinated complex, it is essential for the metal ion to have four equal hybrid orbitals so as to accept four electron pairs from the co−ordinating ligands. This can be achieved in two ways
(i) If one s and three p orbitals hybridizes, four Sp 3 hybrid orbitals are formed and the complex involves Sp 3 hybridization. Due to Sp 3 hybridization, the structure of the complex will be tetrahedral.
(ii) On the other hand, if one d, one s and two p−orbitals hybridizes, four dsp 2 hybrid orbitals result and the complex involves dsp 2 hybridization. Due to dsp 2 hybridization, the structure of the complex will be square planar.
(a) Tetrahedral Complexes
The metal ions like Co 2+ , Fe 2+ , Mn 2+ with Cl− ions as ligand can form tetrahedral complexes.
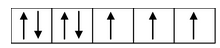
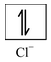
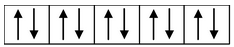
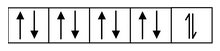
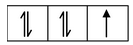
(i) Formation of [CoCl 4 ] 2- : Let us consider the example of the metal ion Co 2+ which in co−ordination with the ligand Cl− forms the complex [CoCl 4 ] 2- . The orbital diagram of the metal ion Co 2+ having configuration 3d 7 can be represented as
|
Co atom in the ground state |
3d
|
4s
|
4p
|
|
Co 2+ ion |
|
|
|
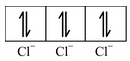
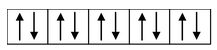
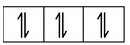
Here, the 3d−orbital of the Co 2+ ion is not available for accepting the lone pair of electrons of Cl− ions. And, since Cl− ion acts as a weak ligand, it can't force the metal electrons to pair up. Hence, one 4s and three 4p−orbitals of this cation hybridize to form four sp 3 hybrid orbitals. The orbital diagram for [CoCl 4 ] 2- can be shown as
|
Formation of [CoCl 4 ] 2- |
|
|
|
|
FOUR ELECTRONS PAIRS DONATED BY FOUR Cl− IONS |
|||
The calculated magnetic moment, µs(cal.) and the experimental magnetic moment, µs (exp.) for the complex [CoCl 4 ] 2- is measured as 3.87 B.M. and 4.30 B.M. respectively which corresponds that it has three unpaired electrons and hence paramagnetic. Therefore, in this case, the 3d−orbitals remain undisturbed and sp 3 hybridization occurs resulting in the tetrahedral structure of the complex. The complex may or may not be coloured.
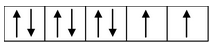
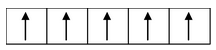
(ii) Formation of [Ni(CO) 4 ]: Oxidation state of nickel in this complex is zero. Its outer electronic configuration is 3d 8 4s 2 . Hence we have
|
Atomic orbitals of Ni in (Z=28) ground state |
3d
|
4s
|
4p
|
|
Hybridized sp 3 orbitals of Ni |
|
|
|
|
sp 3 HYBRIDIZATION |
|||
|
Formation of [Ni(CO)4] |
|
|
|
|
FOUR ELECTRON PAIRS DONATED BY FOUR CO MOLECULES |
|||
sp 3 hybrid orbitals accommodate four pair of electrons from four CO molecules and the resulting tetrahedral complex is diamagnetic due to absence of unpaired electrons. The complex is colourless.
(iii) Formation of [zn(NH 3 ) 4 ] 2+ : Complexes of Zn 2+ are invariably tetrahedral because they involve sp 3 hybridization as shown below
|
Zn atom (Z = 30) in ground state |
3d
|
4s
|
4p
|
|
Zn 2+ ion |
|
|
|
|
sp 3 hybridized orbitals of Zn 2+ |
|
|
|
|
FOUR EMPTY sp 3 HYBRID ORBITALS |
|||
|
Formation of [zn(NH 3 ) 4 ] 2+ |
|
|
|
|
FOUR ELECTRON PAIRS DONATED BY FOUR NH3 MOLECULES |
|||
In the above case, 3d−orbitals are fully occupied, therefore, they cannot participate in hybridization. Four sp3 orbitals can be formed by mixing of one 4s and three 4p−orbitals. The four sp3 hybrid orbitals, accommodate four pairs of electrons from four NH3 molecules. The resulting [zn(NH 3 ) 4 ] 2+ will be tetrahedral and will be diamagnetic as there are no unpaired electrons. The complex would be colourless.
Some other examples of complexes having tetrahedral structures are
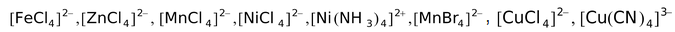 ,
,
 , etc.
, etc.
(b) Square Planar Complexes
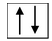
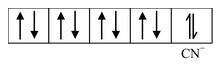
(i) Formation of [Ni(CN) 4 ] 2 - : For understanding the formation of square planar complexes, we consider the example of [Ni(CN) 4 ] 2- ion. We know, the electronic configuration of Ni is 3d 8 4s 2 . But, since the oxidation state of Ni in the complex is +2, its electronic configuration will be 3d 8 and the orbital diagram for the Ni 2+ ion can be represented as
|
Ni atom in the ground state |
3d
|
4s
|
4p
|
|
Ni 2+ ion |
|
|
|
From the experiments, we know that [Ni(CN) 4 ] 2- is diamagnetic and hence the two unpaired d−orbital electrons must become paired.
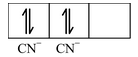
As the CN− ion acts as a strong ligand, it will force the metal electrons to pair up resulting in the dsp 2 hybridization. The orbital diagram for the [Ni(CN) 4 ] 2- ion can be shown as
|
Formation of [Ni(CN) 4 ] 2- |
|
|
|
|
FOUR ELECTRONS PAIRS DONATED BY FOUR
|
|||
Thus, the shape of the complex [Ni(CN) 4 ] 2- ion is square planar. This complex would be colourless.
(ii) Formation of [Cu(Nh 3 ) 4 ] 2+ : Copper in [Cu(Nh 3 ) 4 ] 2+ is in the +2 oxidation state i.e. copper is present as Cu 2 + ion. The formation of [Cu(Nh 3 ) 4 ] 2+ may be explained through hybridization as follows
|
Cu atom (Z = 29) in ground state |
3d
|
4s
|
4p
|
|
Cu 2 + ion |
|
|
|
|
dsp 2 hybridized orbitals of Cu 2 + |
|
|
|
|
FOUR EMPTY dsp 2 HYBRIDIZED ORBITALS |
|||
|
Formation of [Cu(Nh 3 ) 4 ] 2+ |
|
|
|
|
FOUR ELECTRON PAIRS DONATED BY FOUR
|
|||
As [Cu(Nh 3 ) 4 ] 2+ contains one unpaired electron, it is paramagnetic. The complex may or may not be coloured.
Some other examples of square planar complexes include [Cu(Nh 3 ) 4 ] 2- [ptcl 4 ] 2- , [pt (NH 3 ) 4 ] 2+ , etc.
Six Coordinated Complexes
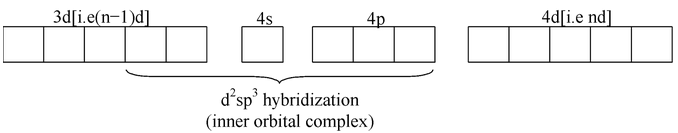
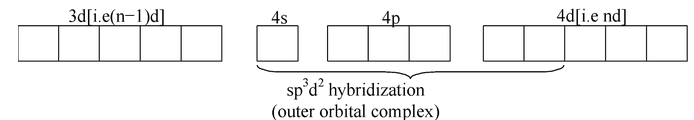
In an octahedral complex, two types of complexes occur i.e. inner orbital complex and outer orbital complex. The distinction between inner and outer orbital complex is based purely on magnetic measurements. In inner orbital complex, (n−1)d orbitals are used for hybridization whereas in outer orbital complex, n d orbitals are used.
Inner and Outer Orbital Complexes
All the complexes containing configuration of metal ion d 0 ,d 1 , d 2 , and d 3 form inner orbital complex i.e. d 2 Sp 3 hybridization. These octahedral complexes contain same number of unpaired electrons as that present in the free metal ion.
If the complex has
d
4
, d
5
,
and
d
6
configuration of metal ion, two types of complexes (i.e. inner and outer orbital complexes) are possible. Most of the complexes of
d
7
,
and
d
8
configuration of metal ion form outer orbital complex (i.e.
Sp
3
d
2
hybridization) but in exceptional cases, a few complexes of this system form inner orbital complexes (i. e.
d
2
Sp
3
hybridization), e.g.
 . The inner and outer orbital complexes may be distinguished by magnetic measurements. Outer orbital complexes are more reactive or labile and consequently ligands may be substituted easily but the inner orbital complexes are sometimes called inert or non−labile and substitution of ligands is fairly difficult.
. The inner and outer orbital complexes may be distinguished by magnetic measurements. Outer orbital complexes are more reactive or labile and consequently ligands may be substituted easily but the inner orbital complexes are sometimes called inert or non−labile and substitution of ligands is fairly difficult.
Usually, we call the ionic complexes as outer orbital complexes and the covalent complexes as the inner orbital complexes. These two classes of complexes are also called spin free and spin paired complexes respectively (by Nyholm) and high spin (HS) and low spin(LS) complexes respectively by Orgel.
Note: A low spin complex ion is a complex ion in which there is maximum pairing of electrons in the orbitals of the metal atom. A high spin complex ion is a complex ion in which there is minimum pairing of electrons in the orbitals of the metal atom.
Octahedral Complexes
(a) Inner orbital complexes
(i) Formation of [Cr(NH 3 ) 6 ] 3+ : The oxidation state of chromium in ion is +3. The electronic configuration of Cr is [Ar] 3d 5 4s 1 . Hence we have
|
Cr atom (Z = 24) in ground state |
3d
|
4s
|
4p
|
|
Cr 3+ ion |
|
|
|
|
d 2 sp 3 hybridized orbitals of Cr 3+ ion |
|
|
|
|
SIX EMPTY d 2 sp 3 HYBRID ORBITALS |
|||
|
Formation of |
|
|
|
|
SIX COORDINATION BONDS FORMED BY DONATION OF SIX ELECTRON PAIRS FROM SIX NH 3 MOLECULES |
|||
Cr 3+ ion provides six empty orbitals to accommodate six pairs of electrons from six molecules of ammonia. The resulting complex involves d 2 sp 3 hybridization and is thus octahedral. The presence of three unpaired electrons in the complex explains its paramagnetic character. The complex may or may not be coloured.
(ii) Formation of ferricyanide ion,:
Oxidation state of Fe in this complex
ion is +3. Hence we have
|
Fe atom (Z = 26) in ground state |
3d |
4s
|
4p
|
|
Fe 3+ ion |
|
|
|
|
d 2 sp 3 hybridized orbitals of Fe 3+ ion |
|
|
|
|
SIX EMPTY d 2 sp 3 HYBRID ORBITALS |
|||
|
Formation of |
|||
|
SIX PAIRS OF ELECTRONS FROM SIX CN− IONS |
|||
The resulting complex involves d 2 sp 3 hybridization and is thus octahedral complex. Further, due to one unpaired electron, it should be slightly paramagnetic which is actually so. The complex may or may not be coloured.
Some other examples of inner orbital complexes are
(b) Outer orbital complexes
(i) Formation of: Oxidation state of cobalt in this complex is +3. Hence we have
|
Co atom (Z = 27) in ground state |
3d |
4s
|
4p
|
4d |
|||
|
ion |
|
|
|||||
|
d 2 sp 3 hybridized orbitals of ion |
|
|
|||||
|
SIX EMPTY sp 3 d 2 HYBRID ORBITALS |
|||||||
|
Formation of[CoF 6 ] 3 − |
|
||||||
|
SIX ELECTRON PAIRS DONATED BY SIX F− IONS |
|||||||
It may be pointed out that F− ion provides a weak ligand field and is unable to pair up the unpaired electrons of the 3d−orbitals. Hence six equivalent hybrid orbitals are obtained by mixing up of one 4s, three 4p and two 4d−orbitals. The highly paramagnetic nature of the complex further confirms the presence of four unpaired electrons. The complex may or may not be coloured.
Some other examples of outer orbital complexes are

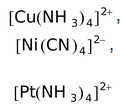 ,
,