
SOLUTIONS
Is matter around us pure of Class 9
SOLUTIONS
A homogeneous mixtures of two or more substance is called a solution. Usually, we think of solution as a liquid that contains either a solid or a liquid dissolve in liquid. However, this is not true. We can also have solid-solid solution, solid-liquid solution, liquid-liquid solution and liquid-gas solution.
For example, lemonade is a solution of sugar, salt and lemon juice in water. These four components of solution do not react with each other since each constituent has its own taste in the lemonade. In other words, lemonade tastes the same throughout, which shows that, there is homogeneity at the particle level in the solution so that, particles of sugar or salt are evenly distributed in the solution.
Components of a solution: A solution has solvent and solute as its constituents which are defined below:
SOLVENT:
The component of solution that dissolves the other component in it (usually present in larger quantity) is called the solvent.
Ex. In the solution of copper sulphate in water, water is the solvent. Similarly, in paints, turpentine oil is the solvent.
SOLUTE:
The component of the solution which is present in small proportion is called solute.
Ex. In the solution of common salt in water, the common salt is solute. Similarly, in carbonated drinks (soda water), carbon dioxide gas in the solute.
Examples of Solutions:
- Solid - Solid solutions : All alloys are solid solutions of metals. Brass is a solid solution of approximately 30% of zinc and 70% of copper. In this solid solution, copper (larger component) is solvent and zinc (smaller component) is solute. Similarly, Bell Metal is a solid solution of 80% of copper and 20% of tin, in which copper is the solvent and tin is the solute.
- Solid - Liquid solutions: Sugar solution is an example, in which sugar is the solute and water is the solvent. Similarly, common salt solution is an example, in which common salt is the solute and water is the solvent. In case of tincture of iodine, iodine is the solute and ethyl alcohol is the solvent.
- Liquid - Liquid solutions: In case of an alcoholic drink, ethyl alcohol is solute and water is solvent. Similarly, in case of vinegar, acetic acid is solute and water is solvent.
- Liquid - Gas solutions: In case of aerated drinks (soda water), carbon dioxide is the solute and water is the solvent.
- Gas - Gas solutions: Air is a homogeneous mixtures of two main gases, i.e., 78% of nitrogen and 21% of oxygen. In this mixture, nitrogen is solvent and oxygen is solute. Similarly, the petrol fed into the engines of automobiles is a mixture of petrol vapour and air.
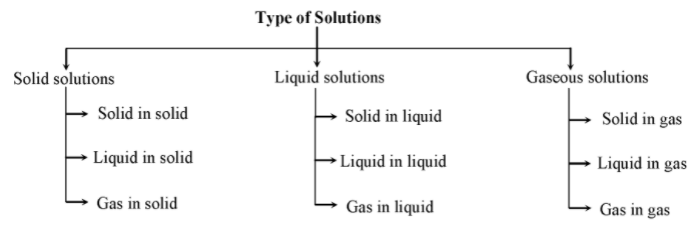
SOLID SOLUTIONS:
In these solutions, solid acts as the solvent, while solute can be either a solid, liquid or gas. Hence solid solutions are further classified into three categories:
- Solution of solid in solid: Metal alloys are the solutions of solids in solids, e.g., brass is a solution of zinc in copper. In this solution, copper (solid) acts as solvent and zinc (solid) acts as a solute.
- Solution of liquid in solid: Hydrated salts (salts containing water of crystallization) such as hydrated copper sulphate (blue vitriol), hydrated ferrous sulphate (green vitriol) etc. are the examples of liquid in solid solutions.
- Solution of gas in solid: Gases adsorbed on the surface of metals like nickel, platinum etc. are the examples of gas in solid solutions.
LIQUID SOLUTIONS:
In these solutions, liquid acts as solvent, while solute can be either a solid, liquid or a gas. Hence liquid solutions are further classified into three categories :
- Solution of solid in liquid: A solution of sugar in water is an example of solid in liquid solution. Here, sugar (solid) is the solute and water (liquid) is the solvent. Similarly, a solution of iodine in alcohol known as tincture of iodine has iodine (solid) as the solute and alcohol (liquid) as the solvent. Thus, it is an example of solid in liquid solution.
- Solution of liquid in liquid : A solution of alcohol in water is an example of liquid in liquid solution. Here, alcohol (liquid) is the solute and water (liquid) is the solvent.
- Solution of gas in liquid : Aerated drinks like soda water etc. are gas in liquid solutions. These contain CO2 (gas) as solute and water (liquid) as solvent.
GASEOUS SOLUTIONS:
In these solutions, gas acts as the solvent while, solute may be a solid, liquid or gas. Hence, gas solutions are further classified into three categories:
- Solution of solid in gas: Iodine or camphor in air are the examples of solid in gas solutions. Here, camphor or iodine (solid) is the solute while air (gas) is the solvent.
- Solution of liquid in gas : Clouds, fog, mist etc. are the examples of liquid in gas solutions. Here, water drops (liquid solute) are dispersed in air (gas solvent).
- Solution of gas in gas : Air is a solution of gas in gas. Air is a Homogeneous mixture of many gases. Its two main gases are oxygen (21%) and nitrogen (78%). The other gases are present in very small quantities. Thus, in air, nitrogen gas (with larger amount) acts as the solvent while other gases (with smaller amount) act as the solute.
The nine types of solutions discussed above are summarized in the following table:
|
Name of the solution |
Solute |
Solvent |
Examples |
|
Solid solutions: 1. Solid in solid
|
Solid |
Solid |
Alloys like brass, bronze, German silver, etc. |
|
2. Liquid in solid |
Liquid |
Solid |
Hydrated crystals such as blue vitriol (hydrated copper sulphate). |
|
3. Gas in solid |
Gas |
Solid |
Gases adsorbed over the surface of metals (such as nickel, palladium, platinum, etc.) under pressure. |
|
Liquid solutions: 4. Solid in liquid |
Solid |
Liquid |
Sugar, common salt or other salts dissolved in water. |
|
5. Liquid in liquid |
Liquid |
Liquid |
Mixture of two miscible liquids such as acetone and water, alcohol and water, etc. |
|
6. Gas in liquid |
Gas |
Liquid |
Aerated drinks (here carbon dioxide is dissolved in water under pressure). |
|
Gaseous solutions: 7. Solid in gas |
Solid |
Gas |
Camphor in air or iodine in air. |
|
8. Liquid in gas |
Liquid |
Gas |
Clouds and fog [here, water drops (liquid) are dispersed in gas (air)]. |
|
9. Gas in gas |
Gas |
Gas |
Air is a mixture of gases like nitrogen, oxygen, carbon dioxide, inert gases, etc. |









