Electronic Displacement In Covalent Bonds
IUPAC & GOC of Class 11
Electronic Displacement In Covalent Bonds
The following four types of electronic effects operates in covalent bonds
- Inductive effect
- Electromeric effect
- Resonance and mesomeric effect
- Hyperconjugation
Inductive Effect (Polar Nature Of Covalent Bonds)
The displacement of an electron (shared) pair along the carbon chain due to the presence of an electron withdrawing or electron releasing groups in the carbon chain is known as inductive effect (I – effect).
-
It is a permanent effect which is transmitted along the chain.
C……> ……C…>….C……>G (G – Functional group)
- This permanent polarity is due to electron displacement due to difference in electronegativities.
-
This effect weakens steadily with increasing distance from the substitution
(electron – withdrawing or electron – donating group) and actually diminishes down after three carbon atoms.
There are two types of inductive effect i.e. – I effect and +I effect.
Negative Inductive effect (⎯ I Effect)
If the substituent attached to the end of the carbon chain is electron withdrawing (X). The effect is called – I effect.
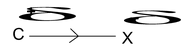
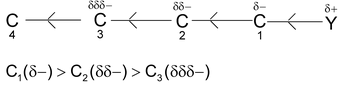
⎯I effect decreases as one goes away from groups (electron attacking)
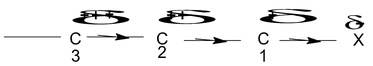
C 1 (δ+) > C2(δδ+) > C 3 (δδδ+) and other third carbon charge is negligible.
⎯I effect is in order.
NO2 > F > COOH > Cl > Br > I > OH > C 6 H 5
Due to ⎯I effect (electron – with drawing nature) electron density decreases, hence basic nature is decreased and acidic nature is increased.
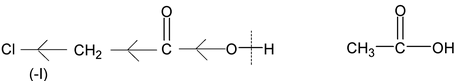
Chloroacetic acid is stronger than acetic acid since Cl shows (-I) effect, electron – density is decreased and O – H bond is weakens causing ionisation of (-COOH) to a greater extent than CH 3 COOH.
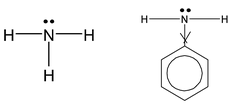
NH 3 is a base due to lone pair on nitrogen. Phenyl group is electron – withdrawing. What happens to electron – density of nitrogen in aniline, Naturally electron – density is decreased. Hence aniline is weaker base than NH 3 .
Similarly acidic nature of phenol is greater than H 2 O due to electron – withdrawing nature of phenyl group.
Positive inductive effect (+I effect):
If the substituent attached to the end of the carbon chain is electron – donating, the effect is called +I effect.
This is due to electron – releasing (Y). It develops a negative charge on the chain.

+I effect also decreases as we go away from group Y (electron – releasing)
So +I effect is in the order of
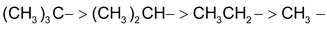
Due to electron – releasing group electron density is increased, hence basic nature is also increased and naturally acidic nature is decreased, thus
|
|
-I effect |
+I effect |
|
Acidic Nature |
↑ | ↓ |
|
Basic Nature |
↓ | ↑ |
Note:
Inductive effect is a permanent effect operating in the ground state of the organic molecules and hence is responsible for high melting point, boiling point and dipole moment of polar compounds.
Electromeric Effect
In presence of an attacking reagent, there is complete transfer of π - electrons from one atom to other to produce temporary polarity on atoms joined by multiple bonds, it is called Electromeric effect.

This effect is temporary and takes place only in the presence of a reagent. As soon as the reagent is removed, the molecule reverts back to its original position. Electromeric effect is of two types, i.e. +E effect and -E effect.
Positive Electromeric effect (+E effect):
When π electrons transfer takes place C to C (as in alkenes, alkynes etc.), it is called positive electromeric effect (denoted by +E )
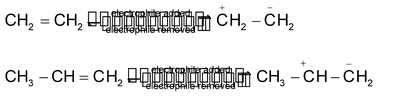
For example, addition of acids to alkenes
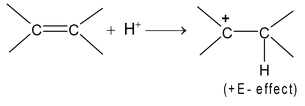
In this, there is also (+I) effect of -CH 3 group which causes π electron transfer from C 2 to C 1 . What do you think in the following case:
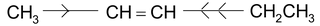
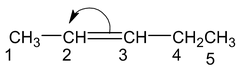
(+I) effect of CH 3 CH 2 - is larger than that of CH 3 - ,hence π electron transfer is from ,C 3 to C 2
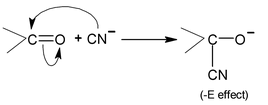
Negative Electromeric effect:
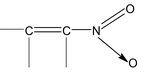
When π- electrons transfer takes places to more electronegative atom (O, N, S) joined by multiple bonds, it is called Negative Electromeric effect (denoted by −E).

for example, the addition of cyanide ion to the carbonyl group.
Resonance & Mesomeric Effect
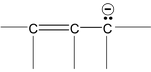
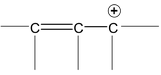
There are many organic molecules which can not be represented by a single lewis structure. In turn, they are assigned more than one structure called canonical forms or contributing of resonating structures. The phenomenon exhibited by such compounds is called resonance. For example, 1, 3 – butadiene has following resonance structure.
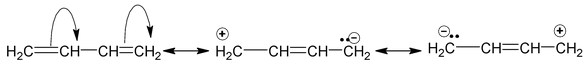
and canonical forms of vinyl chloride are
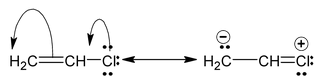
While drawing these canonical forms, the prime thing that has to be kept in mind is that the relative position of any of the atom should not change while we are allowed to change the relative positions of π - bonded electron pair or distribution of charge to other atoms. Also remember that it is not the case that some molecules have one canonical form and some have another form. All the molecules of the substance have the same structure. That structure is always the same all the time and is a weighted average of all the canonical forms. In real sense, these canonical forms have no expect in our imaginations. Now we are in a position to discuss about the conditions necessary for a compound to show resonance. The two essential conditions are
(a) There must be conjugation in the molecule. Conjugation is defined as the presence of alternate double and single bonds in the compound like
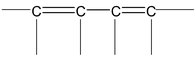
(b) The part of the molecules having conjugation must be essentially planar or nearly planar. The first condition of conjugation is not only confined to the one mentioned above but some other systems are also categorized under conjugation. These are
|
(i) |
|
(ii) |
|
(iii) |
|
|
(iv) |
|
(v) |
|
So, any molecules satisfying both the conditions will show resonance. For example, we consider phenol. The structure of phenol is
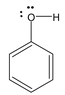
By looking at the structure, it must be clear to you that the compound possesses conjugation of the type

As well as the category (iv) because the lone pairs on oxygen are in conjugation with unsaturated (sp2 hybridised) carbon of the ring. Since, oxygen atom is sp3 hybridized in phenol.
The lone pairs on oxygen are nearly planar with respect to the PZ orbital of carbon linked to oxygen. Thus, both the conditions are fulfilled by phenol, therefore it does show resonance and its resonance structures are represented as
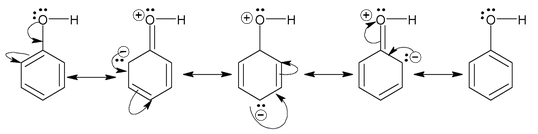
This has to be borne in mind that resonance always results in different distribution of electron density than would be the case if there were no resonance. It is a permanent effect, also referred as mesomeric effect.
Note:
The acidity of phenol can be explained by resonance
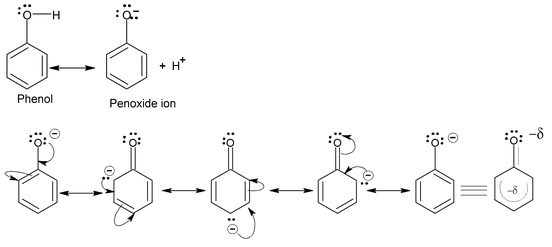
The above structure shows that the phenoxide ion formed is more resonance stabilised than phenol. Hence, the acidity of phenol is explained.
Similarly basicity of aniline can be explained.
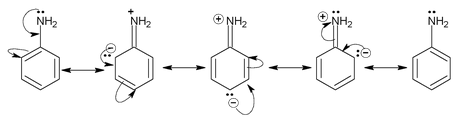
The above structure shows that the lone pair present on N – atom undergoes into resonance and is not available for donation. Hence, the basicity of aniline decreases and is less than aliphatic amine
 .
.
Resonance (mesomeric) effect is of two types.
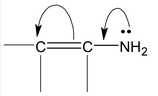
(i) If the atom or group of atoms is giving electrons through resonance, it is called +R or +M effect. For example,
|
|
(+M effect of ⎯NH2 group) |
Other groups that shows +M effect are ⎯NHR, ⎯NR 2, ⎯OH, ⎯OR, ⎯NHCOR, ⎯Cl, ⎯Br, ⎯I etc.
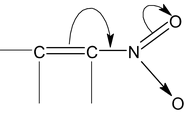
(ii) If the atom or group of atoms is withdrawing electrons through resonance, it is called ⎯R or ⎯M effect. For example,
|
|
(-M effect of ⎯NO2 group) |
Other groups showing ⎯M effect are ⎯CN, ⎯CHO, ⎯COR, ⎯CO2H, ⎯CO 2 R, ⎯CONH 2 , ⎯SO 3 H, ⎯COCl etc.
Now, let us consider resonance in nitrobenzene and its various canonical structures are
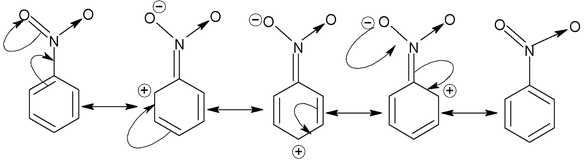
The ⎯NO 2 group in nitrobenzene has ⎯M effect. In general, if any atom (of the group) attached to the carbon of benzene ring bears atleast one lone pair, then the group shows +M effect while if the atom (of the group) linked to the benzene carbon bears either a partial or full positive charge, then the group exhibits ⎯M effect.
In drawing the canonical forms and deciding about their relative stabilities, following rules are give for your guidance.
(i) All the canonical forms must be bonafide lewis structures for example, none of them may have a carbon with five bonds.
(ii) All atoms taking part in the resonance must lie in a plane or nearly so. The reason for planarity is to have maximum overlap of the p – orbitals.
(iii) All canonical forms must have the same number of unpaired electrons. Thus
CH
2
⎯ CH = CH ⎯ CH
2
is not a valid canonical form for 1, 3 ⎯ butadien.
(iv) The energy of the hybrid (actual) molecule is lower than that of any canonical form. Obviously then, delocalization is a stabilizing phenomenon. The difference in energy between the hybrid and the most stable canonical structure is called resonance energy.
(v) All canonical forms do not contribute equally to the actual molecule. Each form contributes in proportion to its stability, the most stable form contributing the most.
(vi) Structures with more covalent bonds are generally more stable than those with fewer covalent bonds.
(vii) Structure with formal charges is less stable than uncharged structures. For charged structure, the stability is decreased by an increase in charge separation and the structure with two like charges on adjacent atoms are highly unfavourable.
(viii) Structures that carry a negative charge on a more electronegative atom are more stable than those in which the charge is on a less electronegative atom. For example,
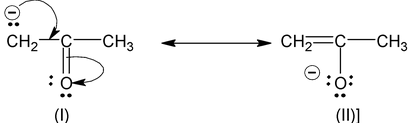
Structure (II) is more stable than (I). Similarly positive charges are best occupied on atoms of low electronegativity
(ix) Those structures in which octet of every atom (expect for hydrogen which have douplet) is complete are more stable than the others with non complete octets. For example,
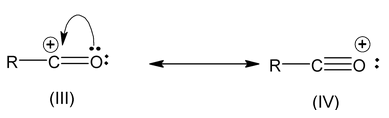
Structure (IV) is more stable than (III).
Resonance Energy:
The difference in energy between the hybrid and the most stable canonical structure is called as resonance energy
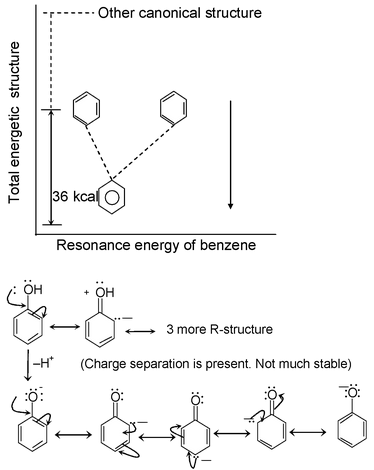
(No charge separation, more stabilization by Resonance)
Hyperconjugation
-
It is delocalisation of sigma electrons.
-
Also known as sigma-pi – conjugation or no bond resonance
-
Hyperconjugation is a permanent effect
Occurrence
Alkene, alkynes
Free radicals (saturated type) carbonium ions (saturated type)
Condition
Presence of α–H with respect to double bond, triple bond carbon containing positive charge (in carbonium ion) or unpaired electron (in free radicals)
Example
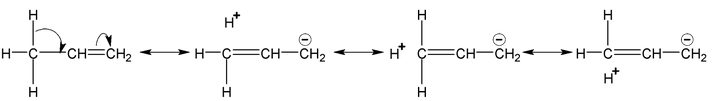
Note:
Number of hyperconjugative structures = number of α-Hydrogen. Hence, in above examples structures i,ii,iii,iv are hyperconjugate structures (4-structures).
Effects of hyperconjugation
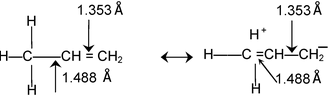
Bond Length:
|
Like resonance, hyperconjugation also affects bond lengths because during the process the single bond in a compound acquires some double bond character and vice-versa. E.g. C—C bond length in propene is 1.488 Å as compared to 1.334Å in ethylene. |
|
Dipole moment:
Since hyperconjugation causes these development of charges, it also affects the dipole moment of the molecule.
Illustration 15. Arrange the following species in increasing order of dipole moment.
Solution:
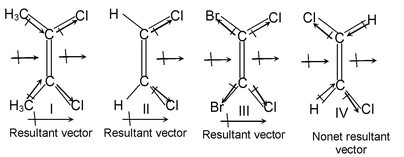
In I, there is addition of vector
In II, there will neither be addition nor subtraction of vector
In III, there is subtraction of vector
In IV, the vectors almost cancel each other. So the increasing order of dipole moment is IV • III • II • I.
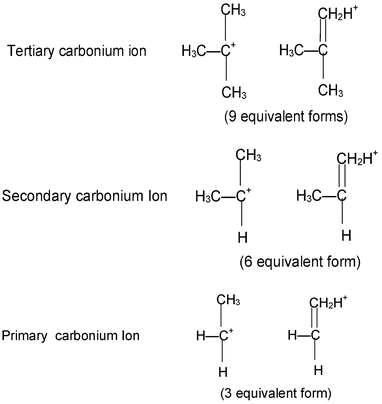
Stability of carbonium Ions:
The order of stability of carbonium ions is as follows
Tertiary • Secondary • Primary
Above order of stability can be explained by hyperconjugation. In general greater the number of hydrogen atoms attached to α-carbon atoms, the more hyperconjugative forms can be written and thus greater will be the stability of carbonium ions.
Stability of Free radicals:
Stability of Free radicals can also be explained as that of carbonium ion
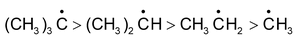
Directive influence of methyl group:
The o,p-directing influence of the methyl group in methyl benzene is attributed partly to inductive and partly to hyperconjugation effect.
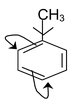
(orientation influence of the methyl group due to +I effect )
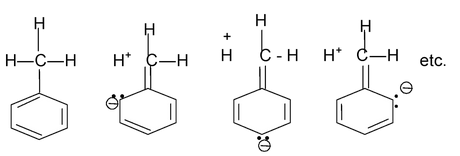
(Orientation influence of methyl group due to hyperconjugation)
The role of hyperconjugation in o,p,-directing influence of methyl group is evidenced by the part that nitration of p-iso propyl toluene and p-tert-butyl toluene from the product in which —NO2 group is introduced in the ortho position with respect to methyl group and not to isopropyl or t-butyl group although the latter groups are more electron donating than Methyl groups
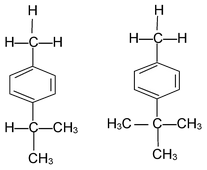
i.e., The substitution takes place contrary to inductive effect. Actually this constitutes an example where hyperconjugation overpowers inductive effect.