
Classification Of Matter
Some Basic Concept Of Chemistry of Class 11
Classification Of Matter
This classification of matter is based upon chemical composition of various substances. According to this matter can be further divided into two types, elements and compounds. Mixtures are also of two types, homogenous mixtures and heterogeneous mixtures.
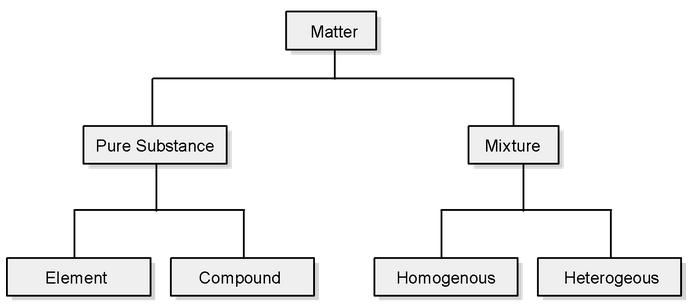
Elements:
The primary stuff present in all the substance is known as element, whose smallest unit known as atom. Total 112 elements are known till date of which 92 are naturally occurring elements rest are results of artificial transmutation. There are 88 metals, 18 non metals and 6 metaloids.
Compound:
A non elemental pure substance is called a compound in which more than one elements atoms are linked by chemical bonds formed due to chemical reaction. The resulting molecule is a electrically neutral particle of constant continuous composition.
Mixture:
Mixtures are the aggregate of more than one type of pure substance whose chemical identity remains maintained even in mixtures. Their constituent ratio may vary unlike compound:
For example – sugar + water = sugar syrup, Gun-powder 75 % KNO 3 10% sulphur + 15% carbon
There are two types of mixture (a) homogeneous (b) heterogenous
(a) Homogeneous mixtures are those whose composition for each part remains constants. For example aqueous and gaseous solution.
(b) Heterogeneous mixtures are those whose composition may vary for each and every part. For example soil, concrete mixtures.




