EMF of the cell
Apr 28, 2022, 16:45 IST
What is Galvanic cell
Galvanic cell is a device which uses a spontaneous chemical reaction to produce electricity. A typical galvanic cell is made up to two half cell, in one of which oxidation takes place and in the other reduction takes place.When we place a piece of zinc in a solution of copper (II) sulphate, the zinc soon become coated with a red - brown deposit of copper, and the blue color of the solution, due to the hydrated copper ion, fades. If enough zinc is present, the solution eventually becomes colorless. These color changes result from the reaction in which zinc is oxidized to Zn 2+ (aq) while Cu 2+ (aq) is reduced to copper. The sulphate ions take no part in this reaction, in which electrons are transferred from zinc to copper. When the reaction is carried out in this way, the transfer of electrons occurs on the surface of the zinc and cannot be directly observed.
But if the oxidation and reduction reactions are separated from each other, the electron transfer can occur through an external circuit. We then have an electrochemical cell that is producing an electric current.
Half Cell and Electrode
A piece of zinc, called the zinc electrode (a solid metal rod connected to the external circuit), is immersed in a solution of zinc (II) sulphate, and a piece of copper, the copper electrode, is immersed in a solution of copper (II) sulphate. The two solutions are kept apart by a porous barrier that permits ions to move from one solution to the other but prevents the two solutions from mixing. A conducting metal wire connects the two electrodes. At the surface of the zinc electrode, Zinc atoms are oxidized to Zn2+ ions, which pass into solution. The electrons lost by the zinc atoms leave the electrode and flow through the wire to the copper electrode. Here they reduce Cu2+ ions, so a deposit of copper forms on the surface of the electrode. The electrode at which reduction occurs is called cathode and the electrode at which oxidation occurs is called anode.The two compartments of the cell are called half cells. The zinc electrode dipping into a zinc sulphate solution is one half-cell, and the copper electrode dipping into a copper sulphate solution the other half - cell.
Any electrochemical cell consists of two half-cells, oxidation takes place in one, and reduction takes place in the other. Neither half cell reaction can take place by itself, because each must be accompanied by another half cell reaction that uses up or supplies the necessary electrons. These electrons constitute the electric current that flows through the external circuit. Not only must the circuit be complete outside the cell, but it must be complete inside as well; that is, ions must be able to move from one electrode to the other. This movement is made possible by separating the half cells with a porous partition (of clay pottery, for example)or a salt bridge.
Salt bridge
It is U - shaped glass tube filled with a jelly like substance, agar - agar, mixed with an electrolytes like KCl , KNO3 , NH4 NO3 , etc. It performs following functions.
- It prevents voltage drops, i.e. prevents junction potential.
- It allows flow of current by completing the circuit, i.e. migration of anion from anode to cathode half cell
- It prevents accumulation of charges and maintains the electrical neutrality of solutions by intermigrations of ions into two half cell reactions.
Characteristics of electrolyte used as salt bridge:
- The electrolyte should be inert and should not react chemically with the electrolyte of either of the two half cells.
- The cation as well as anion of the electrolyte should have same ionic mobility and almost same transport number, viz, KCl , KNO 3 , NH 4 NO 3 etc.
From the direction of the overall spontaneous reaction, we know that Zn gives up its electrons more easily than Cu, so it is stronger reducing agent. Therefore, the equilibrium position of the Zn half-reaction lies further to the right: Zn produces more electrons than does Cu. You might think of the electrons in the Zn electrode as having a greater electron “pressure” than those in the Cu electrode, a greater potential energy (referred to as electrical potential) ready to “push” the electrons through the circuit. Close the switch and electrons flow from the Zn to the Cu electrode to equalize this electron pressure (electrical potential) difference. The flow disturbs the equilibrium at each electrode. The Zn half-reaction shifts to the left to remove the electrons flowing in. Thus, the spontaneous reaction occurs as a result of the different abilities of these metals to give up their electrons and the ability of the electrons to flow through the circuit.
Zinc atoms become ions and attract the attention of water molecules. Now the solvated Zn 2+ (aq) ions diffuse away into solution, thereby exposing a new ‘workface’ of zinc atoms on the electrode.
Water molecules would be attracted by Zn 2+ , a cation of quite considerable charge density, in an ion-dipole interaction.Electrons leave the zinc electrode and make their way round to the Cu/Cu 2+ side of the cell.
The electrons arrive from the zinc side of the cell, and use the copper electrode as a solid conductor to bring them in contact with the species that will act as receivers of electrons, namely the Cu 2+ (aq) ions. The exchange is completed, and the newly formed copper atoms come out of solution and crystallize on to the solid lattice of the electrode material.
Pair of counter-currents run through the salt bridge, anions into zinc side of the cell, and cations into the copper side. In fact the entire current path, both inside and outside the cell, can only be kept open and flowing by the avoidance of build-ups of one sort of charge or another.
EMF of the cell
If you were to place a zinc rod into a solution containing zinc ions, equilibrium would be set up between them. There is tendency for zinc atoms on the surface of the rod to be attracted into the solution. However, they do not enter the solution as atoms, but as zinc ions( Zn 2+ ). In this guise they can be solvated by water molecules. The electrons left behind when a zinc atom is transformed into a positive ion remain on the rod. As a result the region of solution very close to the rod suffers an increase in positive charge (owing to the extra Zn 2+ ions), while the rod carries a layer of negative charge (the electrons left behind).Other metals dipping into solutions of their ions may undergo a similar but opposite change. For them, some of the ions in the solution cling on to the metal and attract electrons out of the rod this leaves the rod with a positive charge. Because the solution near to the rod loses positive ions, it becomes slightly negatively charged.
An electric double layer is set up in both cases. This layer is known as the Helmholtz double layer.
If we call the metal M and we assume that it makes a positively charged ion M n+ , the same equilibrium is involved in both cases:M n+ (aq) + ne - →M(s)
The difference is that for some metals the equilibrium lies to the left (these give extra positive ions into the solution),while for others it lies to the right (these take positive ions out of the solution).Whenever there is a separation of positive and negative charges, we should be able to measure a voltage. In this case there should be a voltage between the electrode and the surrounding solution. At first sight it might seem an easy thing to measure this voltage. All we need is a voltmeter and two pieces of wire. One piece we connect to the electrode, the other we dip into the solution. Unfortunately this idea has a flaw in it. As soon as we dip the metal wire into the solution, another equilibrium is set up. This time it is between the metal from which the wire is made and the ions it gives in solution. We have introduced another Helmholtz double layer. The best we can do now is to measure the difference in voltage between the two double layers. This is an unavoidable state of affairs; we cannot directly measure the voltage between the Helmholtz double layer.
This is unfortunate because if we could measure the voltage it would tell us something about how good a metal is at releasing electrons. Metals that release electrons easily are good reducing agents, so by comparing the voltage for each metal we would be able to put them in order of their reducing power.Given that we are bound to measure a voltage between two double layers, the most convenient thing to do is to agree to keep one of them constant and always measure the difference between this one and the others. The system that has been chosen as a standard is the standard hydrogen electrode. We shall refer to this as the S.H.E.
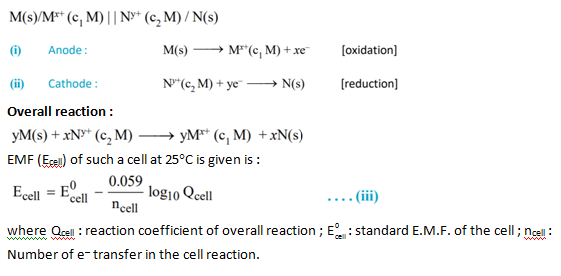
Up to this point, we have focused on cell potentials determined for cells with reactants and products in standard states (
E
); however, cells need not operate at standard state conditions.
We can use the equation for the free energy change of a reaction to derive a relationship that tells us how the cell potential changes as the concentrations and/or temperature change. The free energy change for a reaction is G = G o + RT ln Q
where G o is the free energy change for the reaction with reactants and products at standard state activities (1 molar concentrations, 1 atmosphere pressures, pure solids or liquids), G is the free energy change under some other set of conditions, and Q is the reaction quotient for the reaction at the second set of conditions. Because G = − nFE Cell and G o = − nFE o Cell , we can write