
Formula For Root Mean Square Speed of Gas Molecules
May 01, 2022, 16:45 IST
Root mean Square (C rms ) speed :
The fraction of molecules having either very high or very low speeds is very small. Majority of molecules have speed near to C rms in the middle of the range of molecular speeds. The total area under the curve in Figure is a measure of total number of molecules in collection.
It is denoted by C rms = root square mean value
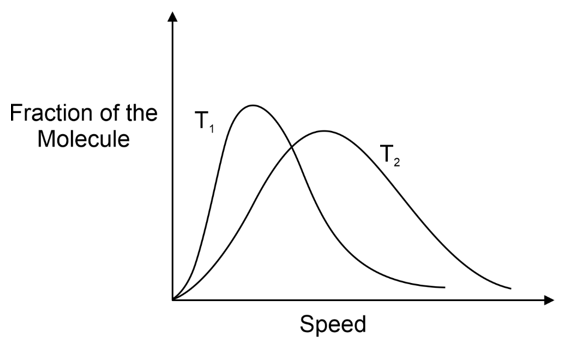
Since, rise in temperature raises the kinetic energy of gas molecules, it follows that fractions of molecules having lower speed range decreases and fraction of molecules having higher speed range increases. Also the curve at higher temperature has its u mps shifted to higher value but the corresponding fractions of molecules have decreased. Thus in general, the distribution of speeds is wider at higher temperature than at lower temperature as shown in figure (T 2 > T 1 )
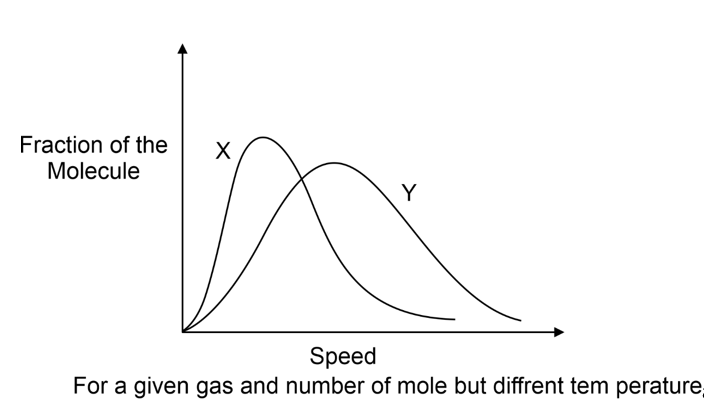
Distribution of molecular speed also depends upon the mass of gas molecule. At similar temperature, a heavier gas molecule has a narrow distribution of speeds than a lighter gas molecule as shown in the following diagram (M X > M Y ):
As per kinetic theory of gases, each molecule is moving with altogether different velocity. Let ‘n’ molecules be present in a given mass of gas, in which n1 molecules have a speed of v 1 , n 2 molecules have a speed of v 2 , ,n n molecules have a speed of v n .
Root mean Square (C rms ) speed formulas
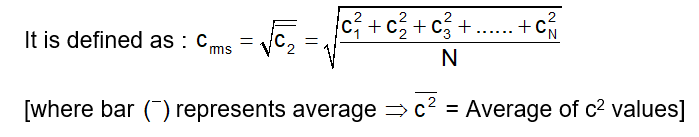
Since the distribution is continuous, we can write :
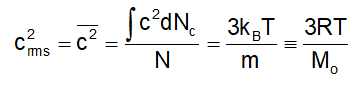
Where M o = Mol. Mass of the gas (in kg)
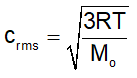
Derivations of Root mean Square
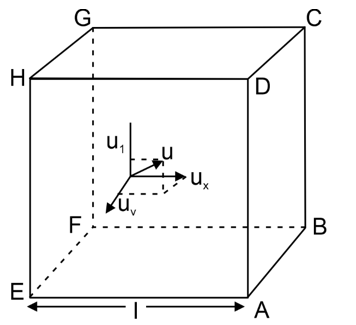
Imagine a cube of edge-length l, containing N molecules, each having a mass of m. Molecules are moving at random in all directions, with speed covering a considerable range of values. The velocity u 1 of any molecule may be resolved into three- component velocities designated as u x , u y and u z ., These are in the three directions at right angles to each other and parallel to the sides of the cube as shown in figure. The component velocities are related by the expression
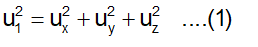
Considering the x-component motion of a molecule, we will have Momentum of molecule before collision with the side ABCD = mu x .
Momentum of the molecule after collision with the side ABCD = –mu x .
Change of momentum of the molecule in a single collision with the side ABCD = |2mu x |. Since l is the edge length of the cube, the molecule has to travel a distance 2l to arrive back at the wall ABCD. The number of collisions per unit time with the wall ABCD will be equal to u x /2l.
The total change of momentum per unit time due to such impacts is
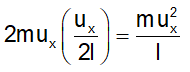
According to Newton’s second law of motion
Force = mass × acceleration
mass x d(velocity)/dt
= d/dt (mass x velolcity)
= d/dt(momentum = rate of change of momentum
Hence, total force due to impacts of a single molecule with the wall ABCD of the vessel is Mu x 2 /l. The area of the wall is l 2 . Hence, the pressure exerted due to the collision of x-component velocity of a single molecule with the side ABCD is
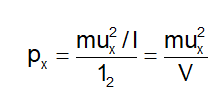
where V is the volume of the vessel
Since each molecule will exert similar pressure, the total pressure exerted on the wall ABCD will be
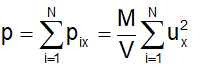
Defining the mean square speed as

Since the directions x, y and z are equivalent, we will also have
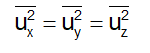
But from Eq. (1), we will have
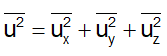
From Eqs (1) and (3), we can write
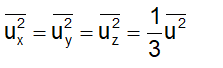
Substituting this in Eq. (2), we get
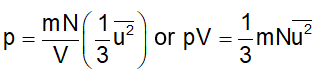
From Maxwell distribution we already know
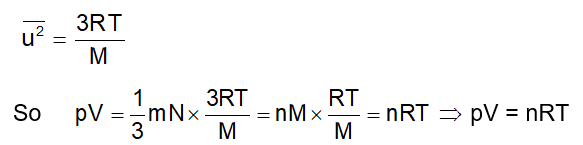
- Average translational kinetic = ½ mu rms 2 = 3/2kT energy per molecule
- Average Translational Kinetic = N A × ½ mu rms 2 = 3/2RT energy per mole
- k(Boltzmann constant) = R/N A = 1.38 × 10 –23 JK –1









